In 2020-2021, we’ll continue to explore the research of Dr. Celia Schiffer, who was recently honored with the William C. Rose Award. However, this year we will explore Dr. Schiffer’s work as it relates to SARS-CoV-2, the virus that causes the current Covid-19 pandemic.
If you are new to the CREST Program in 2020-2021, you might want to learn a bit more about Dr. Schiffer’s work.
Understatement of the year: Covid-19 is impacting EVERYONE across the globe in a variety of ways.
As scientists scramble to understand the virus, where it originated, how it causes disease, and treatments/cures, information (both accurate and speculative) is pouring forth daily. Publishers have eliminated a lot of red tape in getting articles published quickly, and many are providing free access to these articles. There are several sites (including BioRXiv) that will post ‘preprints’ – articles that have not yet undergone peer review. When the article is published, BioRXiv will include a link to the published article – and it has often undergone significant revision since the preprint was posted!
A few words of caution as you delve into the Covid-19/SARS-CoV-2 story:
- Pay attention to the source
- Reliable sources include:
- Published, peer-reviewed articles in scientific journals
- World Health Organization website
- Publications from the US National Academies of Science
- Proceed cautiously:
- Preprint server articles (BioRXiv and the like)
- Best to avoid unless information can be externally verified from scientific sources
- Newspaper, Internet, news feeds
- Pay attention to the date of publication
- Newer research often supersedes outdated research – but all articles published on SARS-CoV-2 have been published in 2020 or 2021!
- Some of your reading may require you to think: ‘This is what we knew in March, this is what we knew in July, and this is what we know now.’
- You might also ask yourself, ‘Why was X announced in April, when we already knew Y in February, but were only told Y in June?’ Often other circumstances besides our scientific knowledge are at play!
- At some point, you’ll have to limit your focus. Spend a little time reading some background information (some good starting points are provided below), then think about what story you want to tell. Some possible projects are suggested, but you may have other great ideas. Please feel free to arrange a Zoom meeting to discuss. The CBM has been collecting a lot of resources, including a list of all the PDB files of SARS-CoV-2 structures and their primary citations.
Coronavirus basics
As you do background reading for your CREST project, you’ll encounter a LOT of terminology. It is almost like learning a new language! To provide a little background – and a ready reference – here is some background information that will help you get started. No need to study this information, just remember where you can go to refresh your memory!
All coronaviruses get their name from the spike proteins on their surface that resemble the spikes on a crown. These viruses are in the baculovirus family. There are four genera: α and β coronaviruses infect mammals, and γ and δ infect birds/fish. SARS-CoV-2, the virus causing the Covid-19 pandemic, is a β coronavirus, along with SARS and MERS, the other two human coronaviruses that cause serious disease. Other coronaviruses that infect humans account for 15-29% of all common colds: 229E and NL63 are α coronaviruses, and OC43 and HKU1 are β coronaviruses (Gussow et al., 2020).
SARS emerged in humans in 2002, causing an epidemic in Southeast Asia and cases identified throughout the world. The virus originated in bats, then was transmitted through civets (called a reservoir species) to humans. Although there was a high fatality rate (9%), the virus was not very adapted to human-to-human transmission (Gussow et al., 2020). The epidemic was contained and the virus fizzled out in 2004. MERS emerged in the Middle East in 2012. MERS also originated in bats but is transmitted to humans through camels. The fatality rate of MERS is 36%, but human-to-human transmission is low (Gussow et al., 2020). MERS continues to circulate in the Middle East.
SARS-CoV-2 appeared on the scene in December 2019. But where did SARS-CoV-2 originate? In an article published in February, SARS-CoV-2 was identified as being most closely related to two bat coronaviruses: bat-SL-CoVZC45 and bat-SL-CoVZXC21 (Lu et al., 2020). It was thought that the reservoir organism was the pangolin. An article published in July (with data from a wider number of organisms) suggests that SARS-CoV-2 appeared as a recombination event between a different bat coronavirus (RaTG13) and a pangolin coronavirus (Pangolin Guangdong 2019) (Boni et al., 2020). Although the details in the two articles differ, it appears that SARS-CoV-2 originated in bats, but that pangolin was the reservoir species (perhaps where recombination occurred) in transmitting to humans. Phylogenetic studies are helpful in elucidating similarities and differences among human coronaviruses that may help explain differences in transmission, severity and mortality of different viruses. They also provide some insights as to what regions and which proteins to target for developing drugs and vaccines.
Interesting side note: pangolins are anteater-like animals with thick scales that are prized in Chinese medicine.
Although we’ve learned a great deal from past human coronavirus outbreaks, SARS-CoV-2 has been a greater challenge to contain for a variety of reasons:
- It is more readily transmitted from human-to-human
- There is a fairly long incubation period before symptoms set in (2-14 days, with median of 5 days)
- People are contagious for ~2 days before they have symptoms
- Patients have a higher viral load than in other coronavirus infections – meaning that they release more viruses when they breathe and release more viruses in their feces
- A large portion (maybe 50%) of folks who have Covid-19 are asymptomatic
- Symptoms are diverse – from what appears to be a mild cold to a sudden, life-threatening immune response (‘cytokine storm’)
Coronavirus Genome
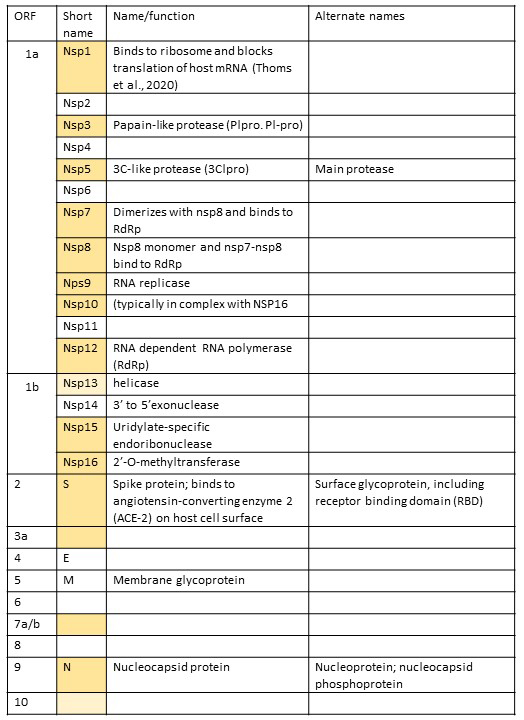
Coronaviruses all have a single positive strand RNA that has a 5’ cap and a 3’ poly-A tail (similar to eukaryotic mRNAs). Their genomes are ~30Kb long (most viruses are in the ~10Kb range, so these are HUGE!!!). The 5’ two thirds of the genome consists of two overlapping reading frames (the second is a -1 frameshift from the first reading frame) which codes for 2 polyproteins that are then cleaved into 16 individual proteins that are involved in viral replication and packaging. Since these proteins aren’t part of the final virus structure, they are called non-structural proteins (nsp), and they are numbered consecutively from 5’ to 3’. The 3’ end of the genome codes for four structural proteins: S – spike protein (binds host cell receptors and is involved in getting virus inside cells; it gives coronaviruses their characteristic ‘crown’ appearance); M – membrane protein (provides virion shape and membrane curvature); E- envelope protein (involved in viral assembly and release); N -nucleocapsid protein (sometimes nucleoprotein, binds RNA genome and helps to package inside virus) (Chen et al., 2020). In the 3’ end of coronaviruses, interspersed among the standard four structural proteins, are unique accessory proteins (different for each virus, though some share common features) that contribute to virus infectivity. The order of all the proteins in the coronavirus genome can be determined because each protein is also assigned an open reading frame (orf) number, beginning at 5’ end of genome.
Below is a list of all the proteins in the SARS-CoV-19 genome, including their name and, where known, their function, starting at the 5’ end of the genome. (This table will be updated as more information becomes available. If you find out more about one of these proteins, please share!) Structures highlighted in goldenrod are in the PDB and have published primary citations. Those in pale yellow are deposited in PDB but are without a published paper to accompany them. These were last updated on September 3, 2020.
Coronavirus Background Reading (in order of publication)
Don’t feel you need to read all these papers! Those with an * were selected for providing a good introduction to the topic. Others are included because they are just interesting! Brief summary is provided in italics. Full reference information is at the bottom of the page.
*April, 2019 (J Alsaadi & Jones, 2019) Nice overview of proteins shared in common among all coronaviruses.
*January 21, 2020 (Chen et al., 2020) Good background paper
*February 3, 2020 (Zhou et al., 2020) Phylogenetic exploration of coronaviruses, highlighting regions of variability (potential drug targets)
March 17, 2020: (Andersen et al., 2020) Interesting paper detailing differences between SARS-CoV-2 and other coronaviruses; arguing that this could not have been a man-made virus
*April 5, 2020 (Sapkota, 2020) Great overview (in bullet form) of proteins (with pdb accession numbers) and genome organization.
*April 10, 2020 (Oldach, 2020) Historical overview of drugs targeting viruses – and what works with Coronaviruses (and why)
*April 16, 2020: (Johns Hopkins Center for Health Security, 2020) Clear, brief background of SARS-CoV-2 structure, genetic makeup, proteins, variability and origin.
*May 8, 2020 (Guy et al., 2020) Brief paper that describes Covid life cycle, which proteins are involved in each step, and potential existing drugs that might be effective in treating (and why).
June 19, 2020 (Servick, 2020) Overview of pros and cons of using phone apps to identify close contacts and notify of exposure.
July 9, 2020 (Thomas, 2020) This is a summary of a pre-print (not yet peer reviewed) from Yale focusing on how the SARS-CoV-2 genome folds into secondary structures. Discussion of role of folding in genome stability and regulatory function
July 10, 2020 (Dehning et al., 2020) This is a great paper describing models of epidemic spread, taking into account efforts to mitigate spread of disease. The paper demonstrates how science is constantly revamping based on collection of more data, and how more data refines our understanding.
*July 31, 2020 (Matheson & Lehner, 2020) This article describes the interaction between the Spike protein and the ACE-2 receptor, which allows the virus to enter the host cell. For those interested in exploring the structure of the Spike protein, this is an especially good paper for background information.
August 2020 (Hart & Halden, 2020) Computer model demonstrating that it is cost effective to test wastewater for presence of COVID-19 as an inexpensive pooling of data from large numbers of people. Note: this screening method is being used on some college campuses to track outbreaks on a dorm by dorm basis, and it has been implemented in some parts of Australia (Ahmed et al., 2020).
Potential Coronavirus Modeling Topics
This is not an exhaustive list – just some idea to consider!
- Comparison of early homology modeling (January 2020) of SARS-CoV-2 spike structure with known crystal structure to determine how accurate the homology modeling was
- SARS-CoV-2 life cycle
- Exploration of one (or more, if they interact together) of the nonstructural proteins involved in viral infection
- Nsp1 protein of SARS-CoV-2 binds to host 40S ribosome, shutting down translation of host proteins. (Thoms et al., 2020)
- Comparison of one of the nonstructural proteins of SARS-CoV-2 with similar protein from SARS or MERS
- Drug target for SARS-CoV-2
- Why are nucleotide analogues used to treat other coronavirus infections ineffective with SARS-CoV-2?
- Compare SARS-CoV-2 +/- bound drug
- Explore a SARS-CoV-2 protein bound to an inhibitor – or, going one step further, evaluate whether the inhibitor interacts only in the substrate envelope
- Select a SARS-CoV-2 protein (structural or nonstructural) and propose a drug structure using the Schiffer substrate envelope approach to reduce development of resistance to drug (advanced topic)
- Developing a vaccine for SARS-CoV-2
- Explore a SARS-CoV-2 protein bound to an antibody
Once your team has narrowed down what you want to model, it’s time to set up a Zoom meeting to discuss your project. (Also feel free to request a Zoom meeting if you need some feedback on narrowing down your focus.) You can share your ideas and get support in refining your topic and selecting a PDB file to explore.
The Schiffer Lab
Dr. Schiffer’s work focuses on designing better drugs that reduce the likelihood that organisms will evolve to become resistant to the drugs. Check out the Schiffer Lab and The Institute for Drug Resistance, of which Dr. Schiffer is a co-founder. We’re excited about the opportunity to work with Dr. Schffer’s lab for a number of reasons:
- Her research takes on a multidisciplinary approach, using principles of organic synthesis, structure-based drug design, crystallography, thermodynamics and enzyme kinetics, virology, co-evolution, and much more – all both facinating and critical in college and career!
- The Schiffer lab studies many diseases, including HIV, Hepatitis C, and Influenza, among others. They study a LOT of different proteins, from HIV-1 protease, to Influenza hemagglutinin, to Dengue NS2B/NS3 protease.
- Dr. Schiffer has LOTS of structures in the PDB from which to choose!!!
- All of her publications (well over one hundred!) are accessible from her website.
Watch the short video below to see Dr. Schiffer explain what her lab studies, and how they are working to counter drug resistance
And, learn more about the lab and the work they do from one of her recent doctoral students, (now Dr.!) Ashley Matthew!
Here are a few videos from the 2019-2020 project that provide some background related to drug design, evolution of resistance, and Dr. Schiffer’s approach to drug design that reduces the development of resistance to the drug.
Intro video (2019-2020):
Drug Design Approaches:
Developing Resistance to Drugs:
Substrate-Envelope Guided Design:
References
Ahmed, W., Angel, N., Edson, J., Bibby, K., Bivins, A., O’Brien, J. W., Choi, P. M., Kitajima, M., Simpson, S. L., Li, J., Tscharke, B., Verhagen, R., Smith, W. J. M., Zaugg, J., Dierens, L., Hugenholtz, P., Thomas, K. V., & Mueller, J. F. (2020). First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: A proof of concept for the wastewater surveillance of COVID-19 in the community. Science of The Total Environment, 728, 138764. https://doi.org/10.1016/j.scitotenv.2020.138764
Andersen, K. G., Rambaut, A., Lipkin, W. I., Holmes, E. C., & Garry, R. F. (2020). The proximal origin of SARS-CoV-2. Nature Medicine, 26(4), 450–452. https://doi.org/10.1038/s41591-020-0820-9
Boni, M. F., Lemey, P., Jiang, X., Lam, T. T.-Y., Perry, B. W., Castoe, T. A., Rambaut, A., & Robertson, D. L. (2020). Evolutionary origins of the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19 pandemic. Nature Microbiology, 1–10. https://doi.org/10.1038/s41564-020-0771-4
Chen, Y., Liu, Q., & Guo, D. (2020). Emerging coronaviruses: Genome structure, replication, and pathogenesis. Journal of Medical Virology, 92(4), 418–423. https://doi.org/10.1002/jmv.25681
Dehning, J., Zierenberg, J., Spitzner, F. P., Wibral, M., Neto, J. P., Wilczek, M., & Priesemann, V. (2020). Inferring change points in the spread of COVID-19 reveals the effectiveness of interventions. Science, 369(6500), eabb9789. https://doi.org/10.1126/science.abb9789
Gussow, A. B., Auslander, N., Faure, G., Wolf, Y. I., Zhang, F., & Koonin, E. V. (2020). Genomic determinants of pathogenicity in SARS-CoV-2 and other human coronaviruses. Proceedings of the National Academy of Sciences, 117(26), 15193–15199. https://doi.org/10.1073/pnas.2008176117
Guy, R. K., DiPaola, R. S., Romanelli, F., & Dutch, R. E. (2020). Rapid repurposing of drugs for COVID-19. Science, 368(6493), 829–830. https://doi.org/10.1126/science.abb9332
Hart, O. E., & Halden, R. U. (2020). Computational analysis of SARS-CoV-2/COVID-19 surveillance by wastewater-based epidemiology locally and globally: Feasibility, economy, opportunities and challenges. Science of The Total Environment, 730, 138875. https://doi.org/10.1016/j.scitotenv.2020.138875
J Alsaadi, E. A., & Jones, I. M. (2019). Membrane binding proteins of coronaviruses. Future Virology, 14(4), 275–286. https://doi.org/10.2217/fvl-2018-0144
Johns Hopkins Center for Health Security. (2020). SARS-CoV-2 Genetics (p. 2). Johns Hopkins Center for Health Security.
Lu, R., Zhao, X., Li, J., Niu, P., Yang, B., Wu, H., Wang, W., Song, H., Huang, B., Zhu, N., Bi, Y., Ma, X., Zhan, F., Wang, L., Hu, T., Zhou, H., Hu, Z., Zhou, W., Zhao, L., … Tan, W. (2020). Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet (London, England), 395(10224), 565–574. https://doi.org/10.1016/S0140-6736(20)30251-8
Matheson, N. J., & Lehner, P. J. (2020). How does SARS-CoV-2 cause COVID-19? Science, 369(6503), 510–511. https://doi.org/10.1126/science.abc6156
Oldach, L. (2020). News: Slipping Past the Proofreader. ASBMB Today.https://www.asbmb.org/asbmb-today/science/041020/slipping-past-the-proofreader
Sapkota, A. (2020, April 5). Structure and Genome of SARS-CoV-2 (COVID-19) with diagram. Microbe Notes. https://microbenotes.com/structure-and-genome-of-sars-cov-2/
Servick, K. (2020). Can phone apps slow the spread of the coronavirus? Science, 368(6497), 1296–1297. https://doi.org/10.1126/science.368.6497.1296
Thomas, L. (2020, July 9). Researchers map RNA structure throughout SARS-CoV-2 genome. News-Medical.Net. https://www.news-medical.net/news/20200709/Researchers-map-RNA-structure-throughout-SARS-CoV-2-genome.aspx
Thoms, M., Buschauer, R., Ameismeier, M., Koepke, L., Denk, T., Hirschenberger, M., Kratzat, H., Hayn, M., Mackens-Kiani, T., Cheng, J., Straub, J. H., Stürzel, C. M., Fröhlich, T., Berninghausen, O., Becker, T., Kirchhoff, F., Sparrer, K. M. J., & Beckmann, R. (2020). Structural basis for translational shutdown and immune evasion by the Nsp1 protein of SARS-CoV-2. Science, eabc8665. https://doi.org/10.1126/science.abc8665
Zhou, P., Yang, X.-L., Wang, X.-G., Hu, B., Zhang, L., Zhang, W., Si, H.-R., Zhu, Y., Li, B., Huang, C.-L., Chen, H.-D., Chen, J., Luo, Y., Guo, H., Jiang, R.-D., Liu, M.-Q., Chen, Y., Shen, X.-R., Wang, X., … Shi, Z.-L. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579(7798), 270–273. https://doi.org/10.1038/s41586-020-2012-7