Campbell University (Buies Creek NC)
Ran Plays an Important Role in Nuclear Transport based on Nucleotide-Dependent Changes in Conformation: the Nucleotide-Free Form of Ran
Team members: Samantha Rodriguez, Emily Stallings and Joshua Schwanke
Faculty advisor: Karen Guzman, Ph.D.
PDB ID: 2mmc
Abstract
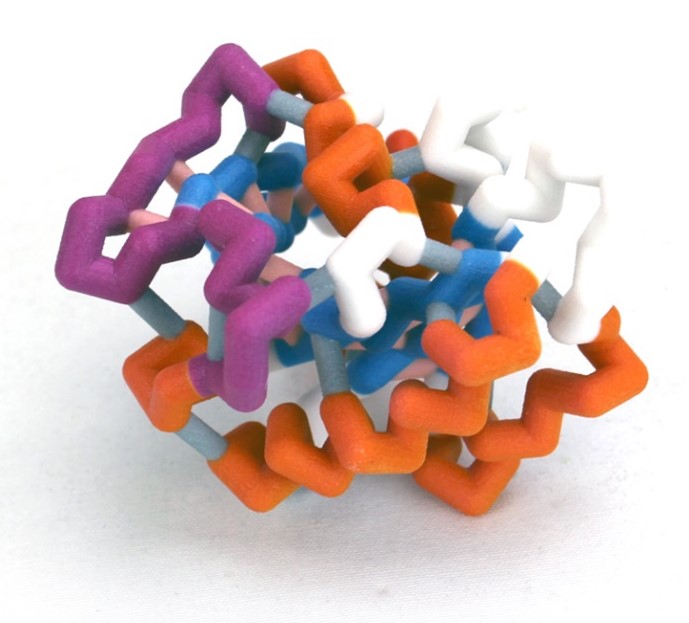 Nuclear transport is a key regulatory process that allows the exchange of large molecules, like proteins and RNAs, between the cytosol and nucleus. The only route to enter or exit the nucleus is by way of nuclear pores. Proteins that are essential to the function of the nucleus are escorted through the pores into the nucleus by shuttle proteins called importins. Since protein synthesis occurs in the cytoplasm, importins pick up their cargo proteins in the cytosol, drop them off in the nucleus, and return to the cytosol for more cargo. Ran is a key player in maintaining this cycle.
Nuclear transport is a key regulatory process that allows the exchange of large molecules, like proteins and RNAs, between the cytosol and nucleus. The only route to enter or exit the nucleus is by way of nuclear pores. Proteins that are essential to the function of the nucleus are escorted through the pores into the nucleus by shuttle proteins called importins. Since protein synthesis occurs in the cytoplasm, importins pick up their cargo proteins in the cytosol, drop them off in the nucleus, and return to the cytosol for more cargo. Ran is a key player in maintaining this cycle.
Ran is a small GTP-binding protein and a member of the Ras superfamily. All members of this family are GTPases that have a common structure which includes five α-helices, six β-strands, and five loops with conserved functions. In nuclear import, Ran plays at least two critical functions. (1) The active form of Ran, RanGTP, initiates the release of cargo proteins from importin inside the nucleus. (2) Ran maintains the direction of nuclear import because the alternate conformations, which result from GDP vs. GTP nucleotide binding, exist as a gradient across the nuclear envelope. This model is part of a multi-model set that illustrates the conserved structure of Ran and the alternate conformations that occur when the different nucleotide ligands are bound. The primary areas involved in the conformational changes are referred to as the Switch I and II Domains. Typically, either GDP or GTP is bound to Ran, but this model illustrates a nucleotide-free form that investigators determined had a conformation most similar to RanGDP.
Campbell University (Buies Creek NC)
Ran Plays an Important Role in Nuclear Transport based on Nucleotide-Dependent Changes in Conformation: the RanGDP Form
Team members: Jake Dressman and James Pericles
Faculty advisor: Karen Guzman, Ph.D.
PDB ID: 3gj0
Abstract
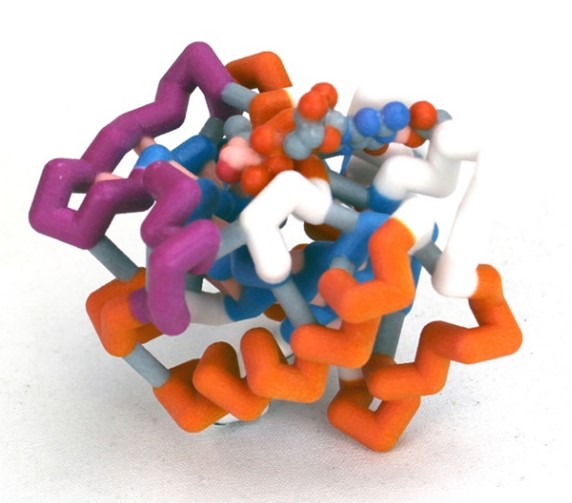 Nuclear transport is a key regulatory process that allows the exchange of large molecules, like proteins and RNAs, between the cytosol and nucleus. The only route to enter or exit the nucleus is by way of nuclear pores. Proteins that are essential to the function of the nucleus are escorted through the pores into the nucleus by shuttle proteins called importins. Since protein synthesis occurs in the cytoplasm, importins pick up their cargo proteins in the cytosol, drop them off in the nucleus, and return to the cytosol for more cargo. Ran is a key player in maintaining this cycle.
Nuclear transport is a key regulatory process that allows the exchange of large molecules, like proteins and RNAs, between the cytosol and nucleus. The only route to enter or exit the nucleus is by way of nuclear pores. Proteins that are essential to the function of the nucleus are escorted through the pores into the nucleus by shuttle proteins called importins. Since protein synthesis occurs in the cytoplasm, importins pick up their cargo proteins in the cytosol, drop them off in the nucleus, and return to the cytosol for more cargo. Ran is a key player in maintaining this cycle.
Ran is a small GTP-binding protein and a member of the Ras superfamily. All members of this family, are GTPases that have a common structure which includes five α-helices, six β-strands, and five loops with conserved functions. In nuclear import, Ran plays at least two critical functions. (1) The active form of Ran, RanGTP, initiates the release of cargo proteins from importin inside the nucleus. (2) Ran maintains the direction of nuclear import because the alternate conformations, which result from GDP vs. GTP nucleotide binding, exist as a gradient across the nuclear envelope.
This model is part of a multi-model set that illustrates the conserved structure of Ran and the alternate conformations that occur when the different nucleotide ligands are bound. The primary areas involved in the conformational changes are referred to as the Switch I and II Domains. This model illustrates the GDP-bound form.
Campbell University (Buies Creek NC)
Ran Plays an Important Role in Nuclear Transport based on Nucleotide-Dependent Changes in Conformation: the RanGTP Form
Team members: Hannah Smith and Garrett Stang
Faculty advisor: Karen Guzman, Ph.D.
PDB ID: 1rrp
Abstract
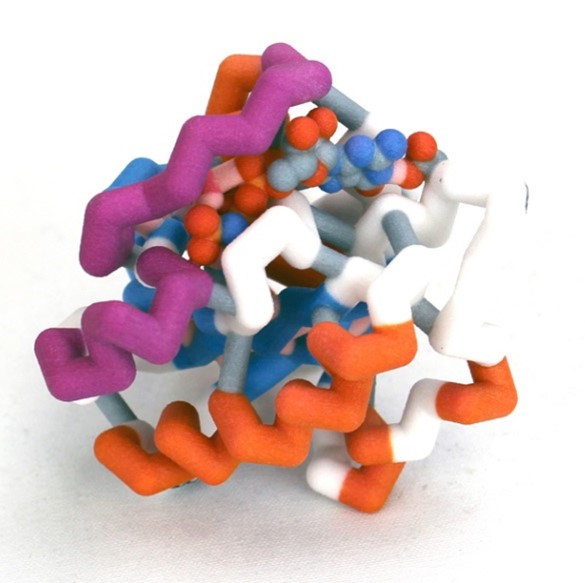 Nuclear transport is a key regulatory process that allows the exchange of large molecules, like proteins and RNAs, between the cytosol and nucleus. The only route to enter or exit the nucleus is by way of nuclear pores. Proteins that are essential to the function of the nucleus are escorted through the pores into the nucleus by shuttle proteins called importins. Since protein synthesis occurs in the cytoplasm, importins pick up their cargo proteins in the cytosol, drop them off in the nucleus, and return to the cytosol for more cargo. Ran is a key player in maintaining this cycle.
Nuclear transport is a key regulatory process that allows the exchange of large molecules, like proteins and RNAs, between the cytosol and nucleus. The only route to enter or exit the nucleus is by way of nuclear pores. Proteins that are essential to the function of the nucleus are escorted through the pores into the nucleus by shuttle proteins called importins. Since protein synthesis occurs in the cytoplasm, importins pick up their cargo proteins in the cytosol, drop them off in the nucleus, and return to the cytosol for more cargo. Ran is a key player in maintaining this cycle.
Ran is a small GTP-binding protein and a member of the Ras superfamily. All members of this family, are GTPases that have a common structure which includes five α-helices, six β-strands, and five loops with conserved functions. In nuclear import, Ran plays at least two critical functions. (1) The active form of Ran, RanGTP, initiates the release of cargo proteins from importin inside the nucleus. (2) Ran maintains the direction of nuclear import because the alternate conformations, which result from GDP vs. GTP nucleotide binding, exist as a gradient across the nuclear envelope.
This model is part of a multi-model set that illustrates the conserved structure of Ran and the alternate conformations that occur when the different nucleotide ligands are bound. The primary areas involved in the conformational changes are referred to as the Switch I and II Domains. This model illustrates the GTP-bound form.