

This Jmol Exploration was created using the Jmol Exploration Webpage Creator from the MSOE Center for BioMolecular Modeling.
This activity explores the tertiary structure of proteins using the Amino Acid Starter Kit. If you do not have access to the kit, images and videos throughout the activity will provide support for your understanding. In this activity, you will build a representational model of a protein (rather than an accurate model of a protein).
The icon seen here indicates that you have a question to answer. You may answer online, then export the answers by clicking on the button at the end of the tutorial. Alternately, you may download and print a copy of the worksheet.
The Amino Acid Starter Kit has several parts:


Proteins in living things are made of only 20 amino acids. These amino acids in humans are either essential, meaning they must come from food; or nonessential, meaning the body makes them. In humans, there are 8 essential amino acids and 12 nonessential amino acids. (Note that some of the nonessential amino acids are essential in older adults and preemies or infants). Different organisms have slightly different numbers of essential and nonessential amino acids.
The primary structure of the protein determines how the protein will fold, although the folding of the tertiary structure is due to side chain interactions alone. The shape of the protein determines the function it will perform. If the shape is incorrect, the protein will function poorly or won't function at all.
You will use the bag of amino acid sidechains. Each represents an amino acid. The colored clip represents the amino acid backbone, and the clear plastic-coated spheres represent the sidechain.

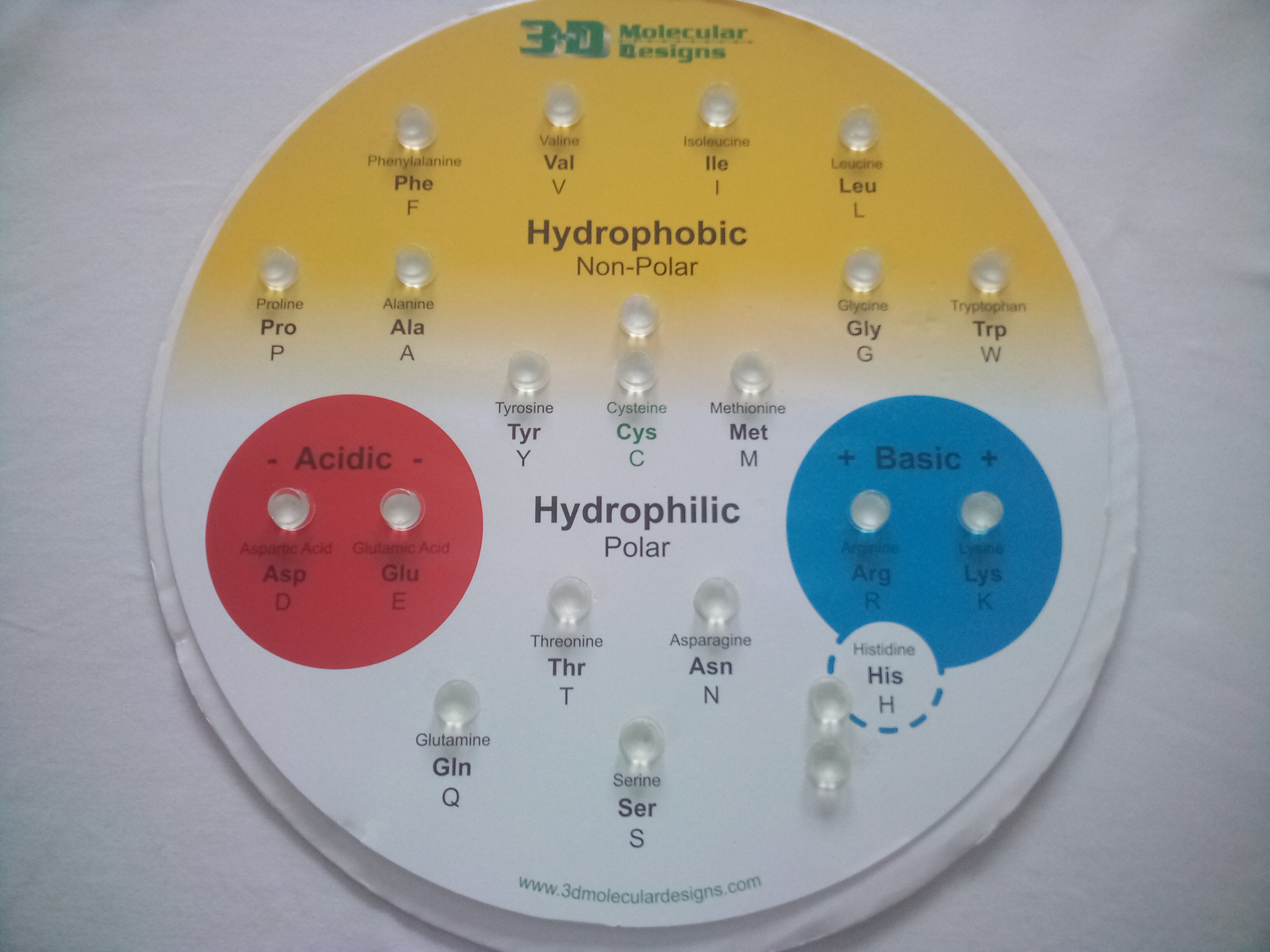
First, group the amino acids based on the color of their backbone clip color.





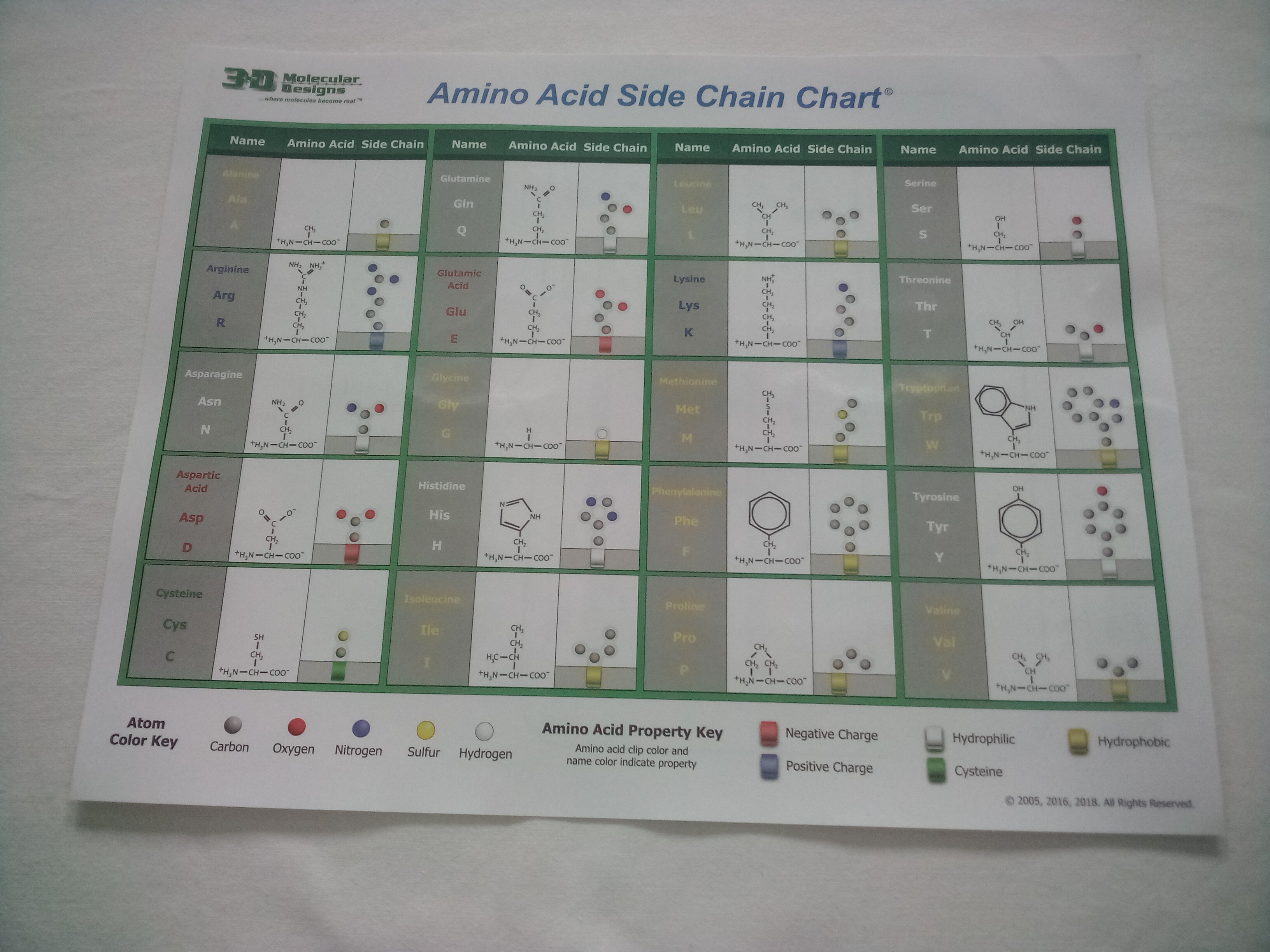
The sidechains (not the clips) use a different coloring scheme, called CPK coloring. (CPK comes from the last initials of the three scientists who developed the coloring scheme.) In CPK, each atom has a specific color:

The buttons below will display each of the amino acids in the window at the right. In these models, the background is colored to indicate the chemical properties of the amino acid, and the backbone is shown in 'soft' cpk colors (light gray carbon, light blue nitrogen and pink oxygen) so you can distinguish it from the sidechains (in 'regular' cpk colors). Each amino acid includes standard 3-letter and 1-letter abbreviations. (This information is also summarized on the Amino Acid Sidechain Chart.)
Look at each of your 'piles' of amino acid sidechains and make an observation about the colors you see and the atoms they represent.
1. What is the predominant color in hydrophobic sidechains?
2. What atom is represented by this color?
3. What additional color appears in acidic sidechains, and what atom does it represent?
4. What additional color appears in basic sidechains, and what atom does it represent?
5. What atoms are found in polar neutral (hydrophilic) sidechains?
6. What atom is found in tryptophan that is not found in other hydrophobic sidechains?
7. Why isn't tryptophan classified as a polar sidechain?
8. Cysteine has green clips because it can form disulfide bonds. Based on what you know about chemical properties, how would you classify cysteine and why?
Place all of the side chains (R groups) in their correct positions on the Chemical Properties Circle according to the chemical properties. The side chains colors- the plastic around the base of each side chain (NOT the atoms) reflect the chemical properties that are found on the circle. Use the Amino Acid Sidechain Chart or Jmol buttons above to help you identify the amino acids. Confirm that the patterns of colors/atoms you described above is what you see when the sidechains are placed on the chart.
Proteins fold spontaneously and very quickly due to the chemical properties of the side chains. Proteins fold in the watery environment of the cell. Predict which side chains will interact with which others.
9. Which type of side chains will be on the inside of most proteins? Why?
10. Which side chains will be attracted to the negatively charged side chains? Why?
11. Which side chains will be attracted to the positively charged side chains? Why?
12. Which side chains will be attracted to the polar neutral side chains? Why?
13. Will the final shape of the protein be a high energy state or a low energy state for all the atoms in the structure? Why?
Unwind the toober. Notice there is a blue and a red end cap. The blue end cap represents the N-terminus (the amino end) and the red end cap represents the C-terminus (the carboxyl end). Since proteins are synthesized from the N terminus to the C terminus, the N terminus is the 'beginning' of the protein.
Select a methionine from the chemical properties circle and place it on the clip closest to the blue end cap. All proteins start with methoinine and protein synthesis begins at the N-terminus of the protein.
https://youtu.be/_h7KgfA7Yds
Select 14 other sidechains:
Mix the sidechains in a pile and randomly distribute them along the toober.

14. What level of protein structure is displayed in the sequence of amino acids on the toober?
15. Where does that information originate in a cell to make this protein?
Fold your protein following basic rules of chemistry. The protein is folding in the watery environment of the cell, so begin by placing all the hydrophobic sidechains on the inside. Remember, proteins fold in three dimensions, so create a three-dimensional structure.

Next, polar sidechains should be on the surface of the protein. Try to arrange these to hydrogen bond with each other. (Some residues might interact with other molecules in the cell, such as substrates or other proteins.) You may have to go back and adjust the hydrophobic residues after arranging the polar residues.
Then arrange acidic and basic sidechains on the outside. The positive and negative side chains are attracted to each other. These should be touching each other.

Finally, arrange the cysteine sidechains so that they can form a disulfide (S-S) covalent bond. These sidechains should touch each other.
Then go back and adjust the hydrophobic side chains again – they might have moved while you were folding the others. You need to find a shape where all the correct side chains are in the correct places both on the protein and in relationship to each other. Adjust as needed – don't be frustrated. This final shape is the tertiary structure of the protein.
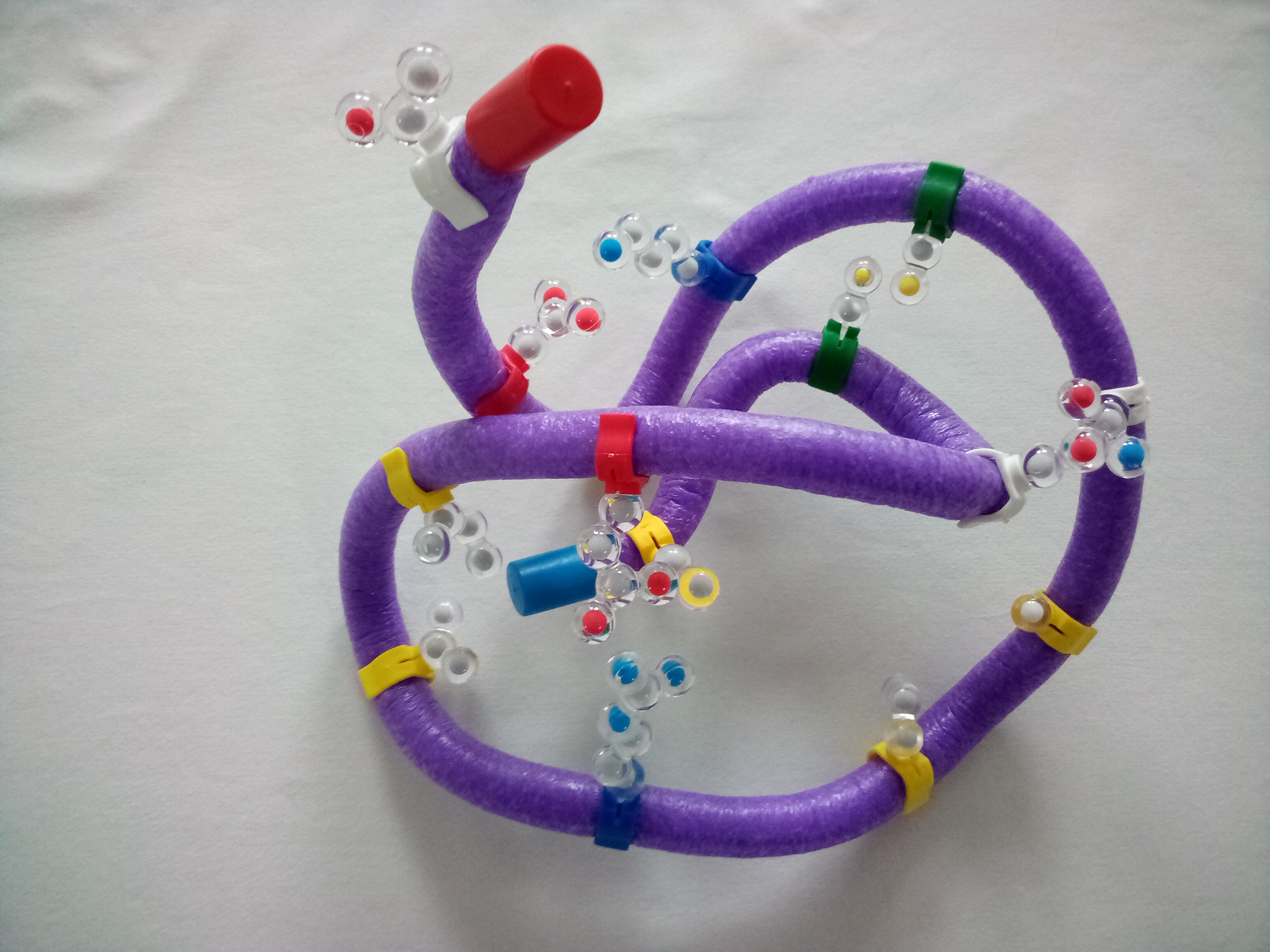
16. As you added each new set of chemical conditions (hydrophobic, positive and negative, polar, and S-S covalent bond) – what happened to the folding and your structure? Why?
17. Does your protein look like any of the others in the class? Why or why not?