

This Jmol Exploration was created using the Jmol Exploration Webpage Creator from the MSOE Center for BioMolecular Modeling.
This activity explores biological macromolecules and their building blocks. Buttons throughout the activity will generate molecular structures in the Jmol window at the right. You may rotate molecules using 'click and drag' in the Jmol window.
This activity uses models from the Molecules of Life collection. If you do not have access to the models, videos and images will guide you through the activity.
This icon indicates you are to provide a response. Although you may enter your responses in the activity, then download a file with your answers at the end of the tutorial, some of the questions require you to complete a table or circle part of an image. You may also download and print a paper copy of the worksheet. Question numbers are included to allow you to easily transition from the tutorial to the worksheet.
Macromolecules are very large molecules that are made up of small molecules that are joined together.
Macromolecules are often called polymers, meaning 'many parts.' Those small molecules that are the building blocks or subunits of macromolecules are called monomers, which means 'one part.'
Polymerization is the process of linking monomers together to form a polymer:
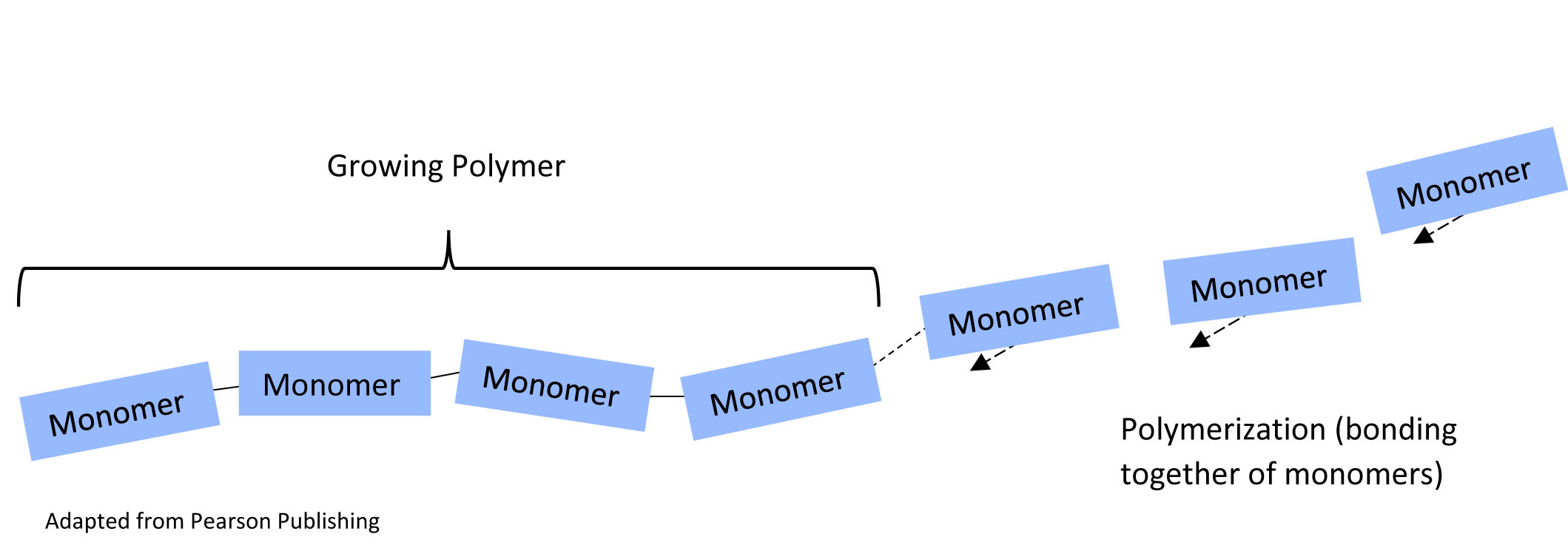
When you click on any of the Jmol buttons in this tutorial, an interactive structure will appear in the Jmol window at the right. You may click and drag with your mouse button to rotate the image. If you hover over an atom with your cursor, a small pop-up will identify the atom using standard chemical symbols. Check it out with the button below, showing the subunits of a polymer.
segment of polymer, with alternating monomers in blue and orange1. How many monomers are already in the growing polymer chain? Hint – do NOT include the monomers with arrows.
2. What are the monomers on the far right (there are three of them) doing?
3. When all the monomers are added, how many monomers will the polymer chain contain?
Monomers polymerize through reactions called condensation reactions. Condensation reactions are also called dehydration reactions, because the new covalent bond formed on the polymer results in the loss of a water molecule.
In a condensation reaction, a monomer is added to the growing chain (monomer in) and water is released (water out).
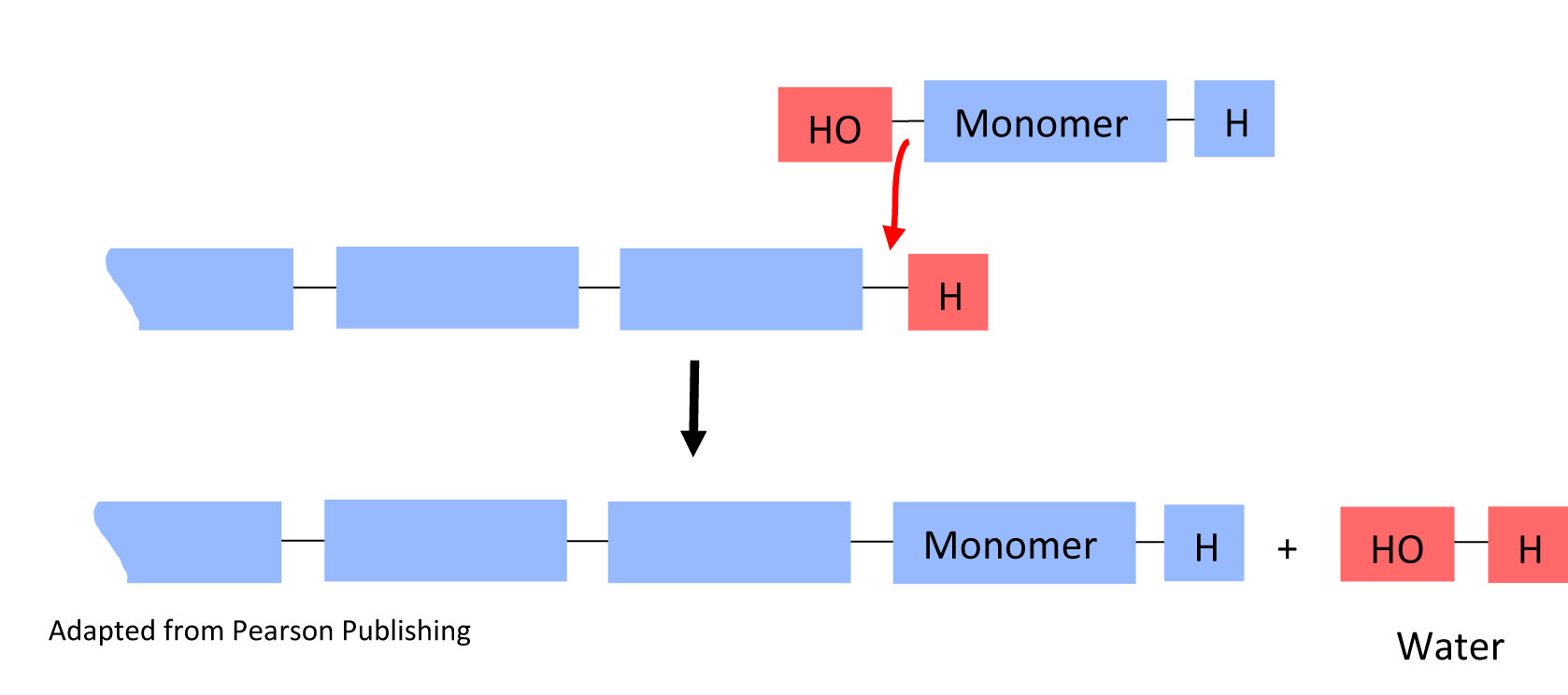
4. In the above condensation reaction, circle the water molecule that is eliminated when the monomer joins the polymer chain in both the top reaction and the bottom result.
The reverse reaction can also occur. Polymers can break down into monomers. This type of reaction is called a hydrolysis reaction. In this reaction, water reacts with the bond linking the monomers, and one monomer is separated from the polymer chain.
In a hydrolysis reaction, a water molecule is added (water in) and a monomer is released from the polymer(monomer out).
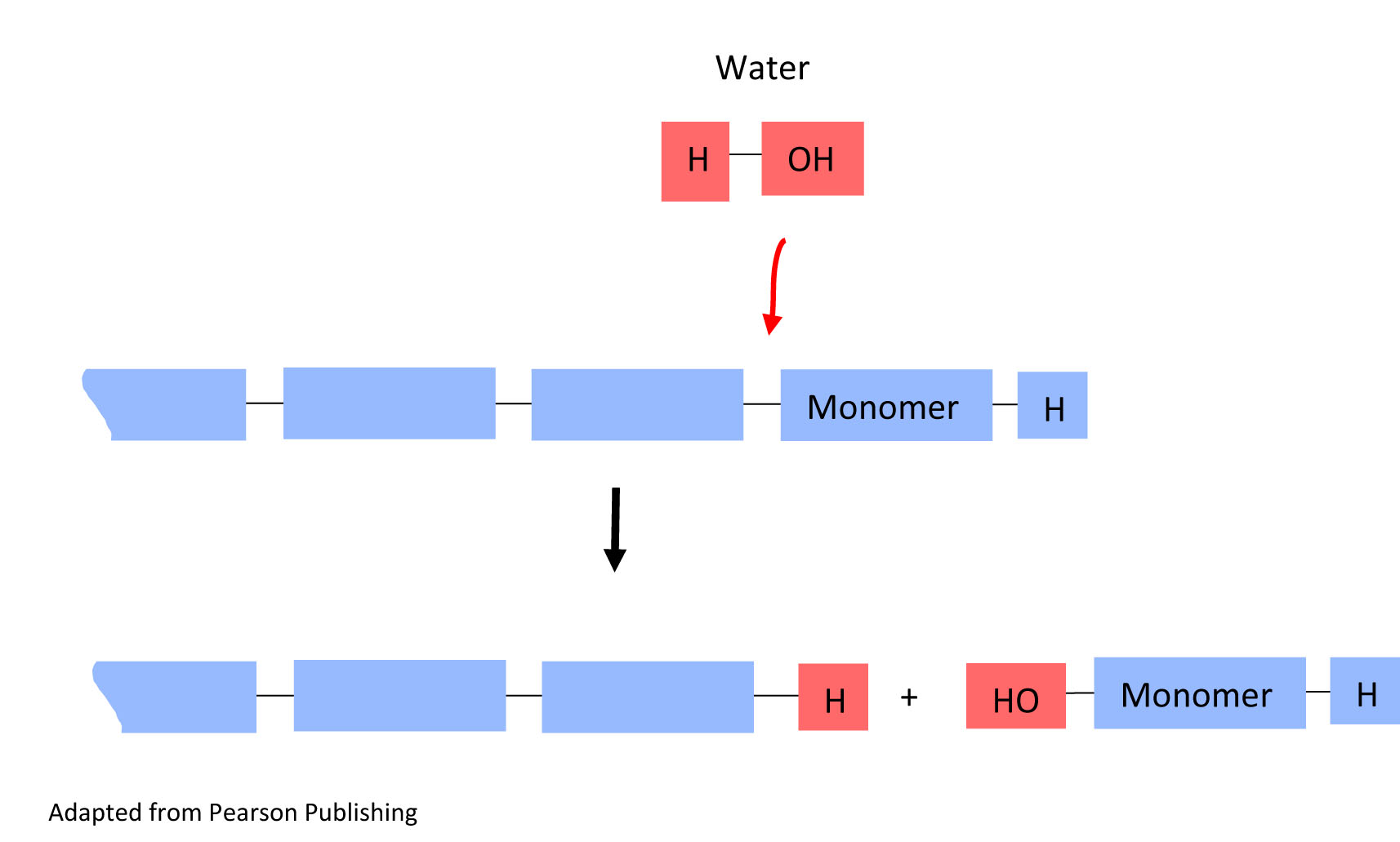
5. In the hydrolysis reaction above, circle the water molecule that is being added to break the monomer unit off the polymer chain in both the top reaction and the bottom result.
In the next few weeks of BIO 100, we will begin talking about macromolecules. There are four classes of macromolecules that are essential for life.
Water, while not a macromolecule, is also essential for life. As you can see in the reactions above, water is essential for the polymerization and for the hydrolysis of these macromolecules.
In this section, you will explore the structure of macromolecules - the large structures of life. Each section includes information about the macromolecule and the monomers used to build it, as well as a short video showing models of both a representative monomer, and a polymer composed of numerous monomers. Each macromolecule has one monomer colored in green. 'Macro' means 'big' - and you'll see that each macromolecule consists of many monomers!
Although you do not need to memorize these structures, you need to 'read' the structures to complete the table. Some of the structures use chemical shorthand - if there is a bend in the 'stick' structure that is not labeled, it is a carbon atom.
All the monomers contain hydrogen, but these atoms are not always drawn in the chemical structure.
As you work through the sections, you'll be directed to answer questions on your worksheet. (You might wish to preview the questions before you start .) You may also wish to refer to your textbook as you complete the table.
Models in the Molecules of Life set follow a standard color scheme used in biology and chemistry. This is known as the CPK color scheme (after the last initial of three scientists who developed the scheme). The colors are as follows:

All of the models in this set are magnified 20 million times (20 x 106 times).
6. An 'average' cell is about 10 μm (1 μm is 10-6 m) in diameter. What would be the diameter of the cell (in meters) if it is magnified to the same scale? Give an example of something that you can relate to that is about the same size.
The monomers of carbohydrates are called monosaccharides. Monosaccharides are energy-rich molecules that provide fuel for your body. Glucose is a monosaccharide that plants build from carbon dioxide and water and energy from the sun in a process called photosynthesis. The energy in glucose is released when organisms break down food during cellular respiration. This energy is captured as ATP and used to power life processes.
glucoseComplex carbohydrates (polysaccharides) consist of many monosaccharide (simple sugar) molecules joined together. Plants use glucose monomers to build two different linear polymers: either starch or cellulose. You, as a human being, have an enzyme (protein) that cuts off the individual glucose monomers of starch - but not cellulose - so that glucose can be oxidized to release its energy. When you eat a meal, some of the glucose is stored in a branched polysaccharide called glycogen. Glycogen rapidly releases glucose to provide energy.
7. What is the monomer of carbohydrates?
8. What elements is it made of?
9. Where are the carbon atoms located in the monomer?
10. Where are the oxygen atoms located in the monomer?
11. What is the name of the specific monomer that makes the specific polymers shown?
12. What process do plants use to make that monomer?
13. What is that monomer used for in both animals and plants?
14. Where are the carbon atoms located in the polymers?
15. Where is the oxygen atom located in the polymers?
16. Complete the following table about carbohydrate polymers.
| Name of Polymer | Structure | Purpose | |
| Plant Polymer |
|
||
| Plant Polymer | Protection as outer part of plants Not easily degraded |
||
| Animal Polymer |
|
The building blocks of cell membranes are phospholipids. They are not technically monomers because they are not connected covalently. Phospholipids are unusual molecules with two distinct properties. One portion of the molecule is a long water-avoiding (hydrophobic) carbon tail. The other portion is a water-seeking (hydrophilic) head group containing phosphate. In your cells, phospholipid molecules spontaneously assemble into a lipid bilayer with the hydrophobic carbon tails buried and hidden from the polar water molecules in the cell. The polar phosphate portion of phospholipids is on the surface, exposed to water.
phospholipidPhospholipid bilayers form cell membranes to separate the inside of your cells from the outside. The hydrophobic nature of a phospholipid bilayer prevents the free movement of most small molecules into and out of your cells. Proteins embedded in cell membranes allow ions and small molecules to pass through the membrane. Each protein has a specific shape that allows it to transport a specific molecule - such as water - into or out of the cell.
section of plasma membrane17. What is the building block of cell membranes?
18. What elements is it made of?
19. Where are the phosphorus atoms located in the monomer?
20. Where are the oxygen atoms located in the monomer?
21. Where are the carbon atoms located in the monomer?
22. What is the unique characteristic of this monomer?
23. Define and identify the part of the phospholipid that is hydrophobic.
23. Define and identify the part of the phospholipid that is hydrophilic.
24. How do these characteristics help make the cell membrane?
25. Where are the phosphorus atoms located in the polymer?
26. Where are the oxygen atoms located in the polymer?
27. Where are the carbon atoms located in the polymer?
28. Can small molecules move easily in and out of the cell through the cell membrane – why or not?
29. What other molecules are found in the cell membrane and what is their job?
Amino acids are the monomers of proteins. Twenty amino acids are used to construct proteins. Each amino acid shares a common backbone with an amino group and a carboxylic acid group (hence the name 'amino acid'). But each amino acid has a unique sidechain, with a unique shape and unique chemical properties. The amino acids are joined together through their common backbone atoms in a unique sequence - specified by your DNA - that spontaneously folds into a compact shape following basic principles of chemistry and physics.
In the Jmol animation of asparagine below, several features shared by all amino acids are highlighted. The flashing purple region is the backbone amino group, and flashing yellow region is the backbone carboxylic acid. Finally, all backbone atoms, including the amino group, the carboxylic acid group and the central a-carbon to which they are attached, is highlighted in green.
asparagineThese linear chains of amino acids are called proteins. Your body produces approximately 100,000 different proteins, each with a specific sequence and a specific shape that allows it to perform a specific function. Aquaporins are channel proteins that facilitate the selective diffusion of water in single file across a cell membrane. Thirteen variants of aquaporin have been identified in humans. Each is composed of 250-300 amino acids. Two polar asparagine amino acids located near the center of the channel guide the water molecules rapidly through the membrane.
aquaporin30. What are the monomers?
31. What elements do they contain?
32. What is the specific monomer shown?
33. How many types of these monomers are used to build the polymer?
34. What is different about each?
35. What is the name of a general polymer?
36. What is the name of this specific polymer?
37. Where does the information to build the polymer come from?
38. What is the function of this polymer?
Nucleotides are the building blocks for your DNA and RNA> There are four nucleotides in DNA, each composed of a base - adenine (A), thymine (T), guanine (G) or cytosine (C) - a deoxyribose sugar and a phosphate group. In RNA, the deoxyribose is replaced by ribose, and the thymine base is replaced by uracil. In both DNA and RNA, enzymes called polymerases join the monomer nucleotides together through their common sugar-phosphate backbone to make long polymers. Cells use the unique sequence of nucleotides in DNA as a code to build the correct sequence of amino acids in a protein.
In the Jmol animation of adenine, the sugar-phosphate backbone common to all nucleotides is highlighted as follows: the phosphate group flashes purple, then the sugar group flashes orange, and finally, the sugar-phosphate backbone flashes in green."
adenosineNucleic acids such as deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) are polymers composed of nucleotides joined by their shared sugar-phosphate backbones. DNA is a long, linear polymer of the nucleotides A, G, C, and T. The sequence of these four nucleotides in your DNA specifies the sequence of amino acids in your proteins. The two complementary strands of DNA wrap around each other to form a right-handed double helix. This double helix contains complementary A-T and G-C base pairs. In 2001, researchers determined the exact nucleotide sequence of the 3.2 billion base pairs of the human genome.
DNA39. What are the monomers called?
40. What elements do they contain?
40. What elements do they contain?
42. What is the specific monomer shown?
43. How many of the monomers are there and what are their names?
44. What are the two polymers that these monomers can make?
45. What are the differences between these two polymers?
46. What does the information from one of these polymers do?
47. How many base pairs are in the human genome?
Life happens in water. The human body is about 70% water. This unique polar solvent provides the medium in which the macromolecules of your cells float. Biomolecules interact with each other in your cells following basic principles of chemistry and physics.
waterWater can exist in any one of three states - gas (vapor), liquid or solid. At 0° Celcius (32° Farenheit) the thermal motion of the water molecules slows to the point that stable hydrogen bonds form between the molecules and an ice lattice forms. Ice floats since ice lattices are less dense than liquid water. This causes lakes to freeze from the top down, insulating the life beneath. Water molecules can form more than a dozen crystalline structures depending on temperature and pressure; however, nearly all ice on the Earth's surface and in its atmosphere is a hexagonal phase noted as ice Ih.
hexagonal ice48. What are the elements in the water molecule?
49. Draw the water molecule and label the elements.
50. Is water polar or nonpolar and why?
51. What are the three states of water?
52. Why does ice float?
53. What is the result of that to living things?
Much like Lego blocks can be connected through a common 'linking mechanism' to build a wide variety of structures, living things contain hundreds of thousands of macromolecules - sometimes called biomolecules because they are associated with life - that are constructed of a limited number of building blocks that are connected through common features between the subunits.
This activity explored four classes of macromolecules: carbohydrates, lipids (the cell membrane is one example), proteins and nucleic acids. In addition to these major classes of macromolecules, cells can combine these four classes into even more complex molecules, including glycoproteins (sugar and protein), lipoproteins (lipid and protein), nucleoproteins (nucleic acid and protein) and lipopolysaccharides (lipid and sugar).
54. This is a table to compare the types of atoms found in each of the molecules of life – polymers. Review the material and complete the information in this table.
| Carbohydrates | Lipids | Proteins | Nucleic Acids | |
| Monomers are called: | ||||
| Example of a polymer: | ||||
| Composed of atoms of: | ||||
| Purpose in the cell: |
55. Which molecule consists of a hydrophobic carbon tail and a hydrophilic phosphate group?
56. What protein helps to transport oxygen from the lungs to peripheral tissues in the body?
57. What are the three components of a nucleotide?
58. What sugar (i.e., carbohydrate) is used by plants to store energy that is captured from the sun in photosynthesis?
59. Which complex carbohydrate is used to store glucose in plants?
60. Which complex carbohydrate is used to store glucose in animals?
61. Models, like the models we used in today's lab are useful, but they are not perfect. A model cannot perfectly replicate a biological molecule or a biological process. Explain one way in which you found one model of your choice useful.