



This Jmol Exploration was created using the Jmol Exploration Webpage Creator from the MSOE Center for BioMolecular Modeling.
This tutorial focuses on organic functional groups found on biochemical and organic molecules. It will also include the types of intermolecular forces that these organic molecules have. These functional groups are what give the molecules their unique characteristics and determine some types of reactions that they undergo.
This first section is a review of electronegativity and polarity, two concepts that are essential when determining properties of functional groups.
All the small molecules used in this tutorial have had their structures determined using X-ray crystallography or diffraction. These structures are deposited in the Cambridge Structural Database (CSD) for use by researchers, educators and students. The CSD accession numbers are provided for each small molecule in this tutorial, in case you want to explore the molecules further.
Throughout the tutorial, clicking on the buttons will launch a Jmol image of the small molecule on the screen at the right.
Once a molecule appears in the Jmol window, wait until the image stops flashing before pushing a button to execute another script. You may spin the protein (left mouse button click and drag), or, if you wish, you may use Jmol commands to explore the molecule further. A Jmol Quick Reference Sheet and Jmol Training Guide are available if you want to play with the images. (WARNING: This can be a lot of fun!)
If you hover over an atom in Jmol, a popup window will identify the atom in the format:
C1 #4
where
C1 is the type of atom.
#4 identifies the atom number in the pdb file.
As you work through the tutorial, the question mark icon will signal that you need to answer a question. With some exceptions, these answers can be inserted directly into the Jmol Exploration, then emailed to your instructor by clicking the button at the end of the exploration. Alternately, you may download a copy of the worksheet.
The most common elements in living things are listed below. Complete the table.
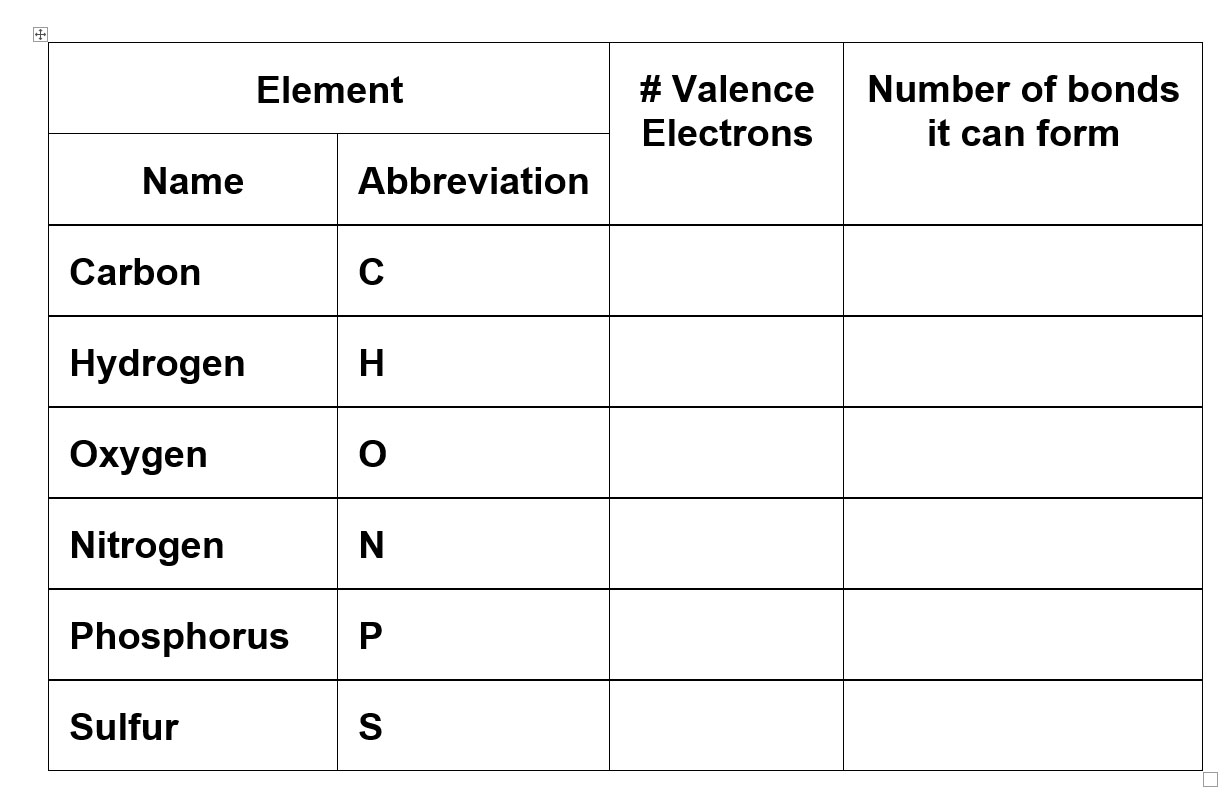
The most electronegative atoms are at the upper right-hand side of the Periodic Table.
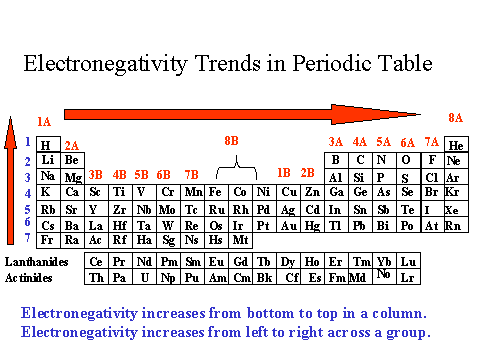
Electronegativity is a measure of how tightly an element holds its electrons (and how much they pull on other atoms' electrons). The more tightly the valence electrons are held the more electronegative the element (atom). An electronegative atom can also remove electrons from other atoms. Fluorine is the most electronegative atom. Oxygen and nitrogen are also electronegative. In biochemistry and organic chemistry we focus on oxygen and nitrogen because fluorine is not commonly found.
Go back to the previous table and choose which of the elements is most electronegative.
Polar molecules have electronegative atoms. These atoms 'pull' the electrons in the covalent bond closer to themselves, resulting in a partial negative charge on those atoms. The atoms on the other side of the bond have a slightly positive charge, as electrons are further away. In water molecules, hydrogen has a partial positive change and oxygen has a partial negative charge due to their differences in electronegativity. Notice that ethane is nonpolar because the carbon and hydrogen have similar electronegativities.
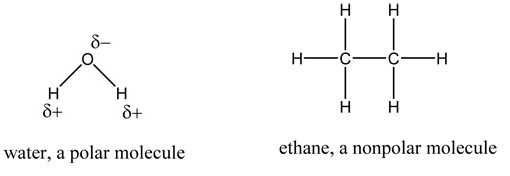
In covalent molecules when one of the atoms is more electronegative than the other(s), the molecule is termed 'polar'. This describes the fact that the electrons spend more time around one element than the other(s).
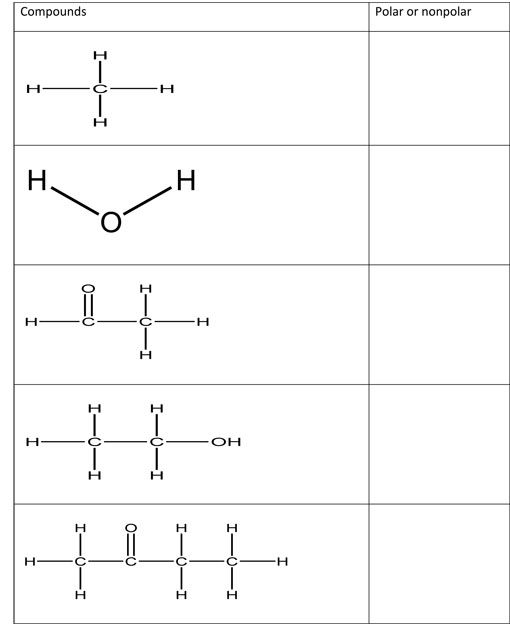
In each compound below, circle the electronegative elements and write polar or nonpolar next to each.
Intermolecular forces are noncovalent and non-ionic attractions between molecules. These forces are responsible for the structure of your DNA and proteins along with boiling points and melting points of all compounds. When these forces are weak, for example, the melting and boiling points are low. When the forces are strong, the melting and boiling points are high. There are three intermolecular forces that we will be studying this semester: hydrogen bonds, dipole-dipole interactions, and (London dispersion forces or induced dipole-induced dipole forces). The last one has multiple names; they are not all exactly the same, but are often used interchangeably. (The term van der Waals is used as a blanket term for these forces in biology, but in chemistry we will be more specific).
The London dispersion or induced dipole-induced dipole forces occur in all molecules and are very weak (the weakest of all the intermolecular forces). They are the ONLY ones that occur between nonpolar molecules. They occur between ALL atoms and molecules – ALWAYS. They occur when the electrons in one of the molecules, for an instant, are around one of the elements more than another and are attracted to another element in another molecule that is the same. Below is an example.
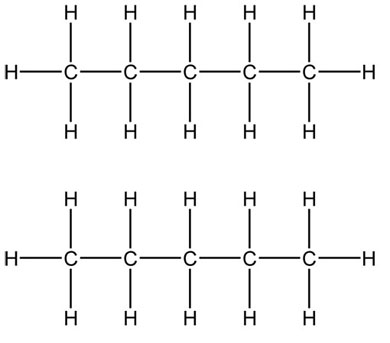
Are these molecules polar or nonpolar?
Would both polar and nonpolar molecules have London dispersion, induced dipole-induced dipole forces or not?
Polar molecules always have London dispersion forces (induced dipole-induced dipole forces) AND can have dipole-dipole forces or attractions occur. These happen when the positively charged end of one molecule is attracted to the negative end of another molecule. These are stronger than the induced dipole-induced dipole (London dispersion forces). (H is not involved in dipole-dipole interactions). Remember that the δ (delta) symbol is used to designate partial charge. Below is an example.
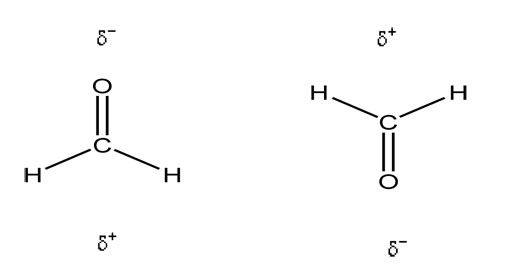
Are the molecules above polar or nonpolar?
Would London dispersion or induced dipole-induced dipole forces also occur? If so why?
Hydrogen bonds are very common and important in all living things. They are a special type of dipole-dipole attractions -- BUT don't occur in all polar molecules. Molecules that Hydrogen bond also have induced dipole-induced dipole (London dispersion forces).
In order for hydrogen bonds to occur, there must be a hydrogen directly attached to an electronegative atom (O, N, halogens). This hydrogen would then hydrogen bond with another electronegative atom (often O or N in living things). They are stronger than either of the other two attractions and are designated with a series of dashed or dotted lines.
Below are examples:
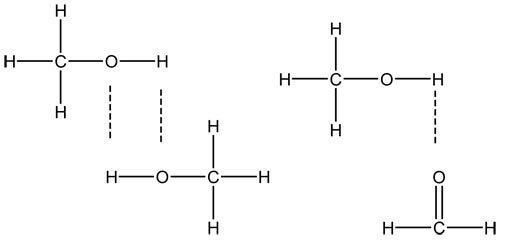
Are these molecules polar or nonpolar?
Would London dispersion or induced dipole-induced dipole forces also occur? If so why?
In the following molecules circle any polar hydrogens.
In the following molecules draw any hydrogen bonds that could form.
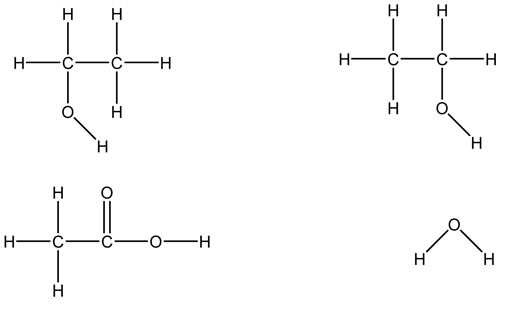
In the following molecules, label the intermolecular attractions: dipole-dipole, hydrogen bonds, and London dispersion or induced dipole-induced dipole.
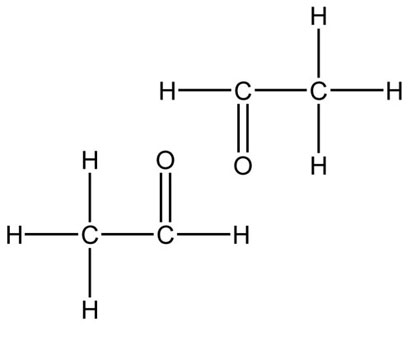
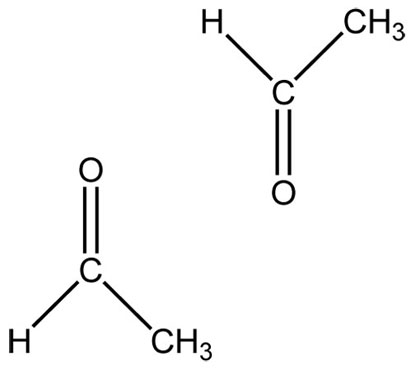
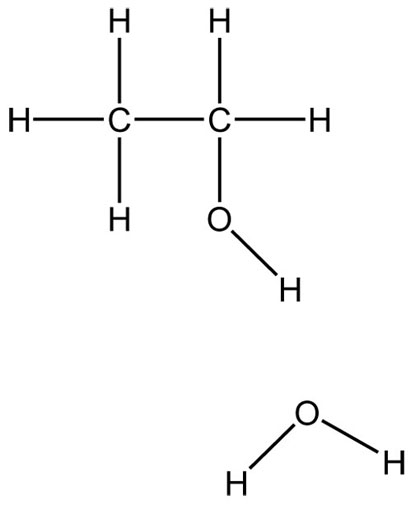
Functional groups are a group of atoms responsible for the characteristic reactions of a family of compounds. The foundational functional group is the alkane. All organic molecules are modifications of the alkane and are named using the alkanes as the basic unit. Other functional groups can be thought of as substituted onto alkanes. Hydrocarbon functional groups, where there are no other kinds of atoms (C, H), include the alkanes, alkenes with carbon-carbon double bonds, and alkynes with carbon-carbon triple bonds. The remaining functional groups have other atoms like oxygen and nitrogen.
When looking at all organic and biochemical molecules the most important thing to look at is the atoms that are NOT carbon and hydrogen. Those are the functional groups and that is where the reactions will occur.
Alkanes are shown below and again, are the basic unit of organic chemistry.
What elements are present in alkanes?
Are these molecules polar or nonpolar?
What types of intermolecular forces will occur between multiple copies of these molecules? Why?
The functional group of the alcohol is -OH. You can also write it as R-OH. 'R' means the rest of the molecule. The R designation is used because the rest of the molecule isn't particularly important, since the reactions occur at the carbon with the functional group. (The rest of the molecules are the carbons and hydrogens NOT connected to the functional group.)
alcohol functional group (green) CSD ID: ETANOLWhat is the molecular formula of the alcohol functional group?
Is the alcohol functional group polar or nonpolar? How do you know?
Are there any polar hydrogens in the alcohol functional group? Why?
Can an alcohol hydrogen bond with other alcohol molecules?
Can alcohols hydrogen bond with water?
The functional group of an aldehyde is H - C = O. This functional group is always at the end of a molecule. That is, the carbon in the functional group is attached to only one other carbon atom in the molecule.
aldehyde functional group (green) CSD ID: GEVREEWhat is the molecular formula of an aldehyde?
Are aldehydes polar or nonpolar? How do you know?
Do aldehydes have any polar hydrogens? WHy?
Can an aldehyde hydrogen bond with other aldehydes?
Can aldehydes hydrogen bond with water? Is the hydrogen on the carbon with the double bonded oxygen polar or nonpolar?
What other type of intermolecular interaction can occur between two aldehydes?
The functional group of a ketone is C = O.
ketone functional group (green) CSD ID: HIXHIF05What is the molecular formula of a ketone functional group?
Is a ketone polar or nonpolar? How do you know?
Do ketones have any polar hydrogens? Why?
Can a ketone hydrogen bond with other ketones?
Can ketones hydrogen bond with water?
What other intermolecular interaction can occur between two ketones?
The functional group of a carboxylic acid is HO - C = O
carboxylic acid functional group (green) CSD ID: ACETAC07What is the molecular formula of a carboxylic acid?
Are carboxylic acids polar or nonpolar? How do you know?
Do carboxylic acids have any polar hydrogens? Why?
Do carboxylic acids hydrogen bond with water?
What other type of intermolecular interaction can occur between carboxylic acids?
The functional group of an ester is O - C = O.
ester functional group (green) CSD ID: BAHSUYWhat is the molecular formula of an ester?
Is an ester polar or nonpolar? How do you know?
Does an ester have any polar hydrogens? Why?
Can an ester hydrogen bond with another ester?
Can esters hydrogen bond with water?
What other type of intermolecular interaction can occur between ester molecules?
The functional group of an amine is NH2.
amine functional group (green) CSD ID: BAZGOYWhat is the molecular formula of an amine?
Are amines polar or nonpolar? How do you know?
Do amines have any polar hydrogens? WHy?
Can amines hydrogen bond with other amines?
Can amines hydrogen bond with water?
The functional group of an amide is H2N - C = O.
amide functional group (green) CSD ID: ZZZMRU02What is the molecular formula of an amide?
Are amides polar or nonpolar? How do you know?
Do amides have any polar hydrogens? Why?
Can an amide hydrogen bond with other amides?
Can amides hydrogen bond with water?
What other intermolecular interaction can occur between two amides?
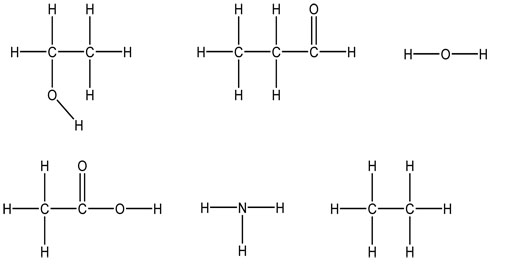
What functional group is shown in example 1?
What functional group is shown in example 2?
What functional group is shown in example 3?
What functional group is shown in example 4?
What functional group is shown in example 5?
What functional group is shown in example 6?
What functional group is shown in example 7?
Which functional groups contain oxygen atoms?
Which functional groups contain nitrogen atoms?
Do you think that having more than one functional group affects the physical and chemical properties of molecules? Why?