



This Jmol Exploration was created using the Jmol Exploration Webpage Creator from the MSOE Center for BioMolecular Modeling.
Fischer projections are a type of shorthand that allows us to draw chiral molecules on a flat piece of paper. They were developed by Emil Fischer. There are a few basic rules to follow when drawing/interpreting Fischer projections.
1) Lines extending horizontally from the chiral center extend out of the plane of the paper towards you.
2) Lines extending vertically from the chiral center extend back behind the plane of the paper, away from you.
3) Each chiral center is considered individually. If two adjacent atoms are both chiral, you may need to rotate the molecule as you view chiral centers to be sure that the attached groups are pointing in the correct direction relative to the plane of the paper.
Let's start with a simple molecule containing a single carbon atom to which 4 different constituents are attached.
simple chiral moleculeNote that in when the molecule above stops spinning, it is properly aligned to draw the Fischer projection. The chiral atom is the gray sphere in the middle. Attached groups that lie horizontally are in front of the gray atom, and groups that lie vertically are behind the gray atom. The Fischer projection is written:
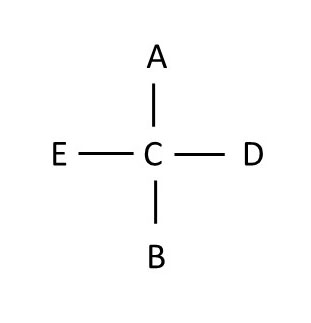
This molecule can be rotated in several ways. Each of the following rotations starts with the previous orientation and rotates to a new orientation. Fischer projections for each rotation are drawn. Note that ALL of these Fischer projections are of the same molecule, and therefore they are all equivalent.
rotation 1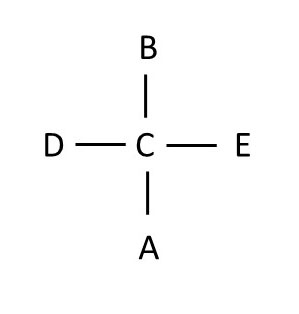 rotation 2
rotation 2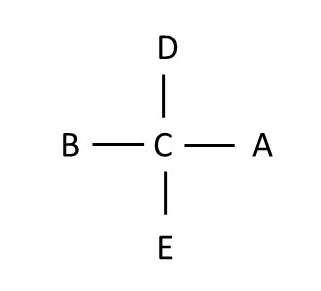 rotation 3
rotation 3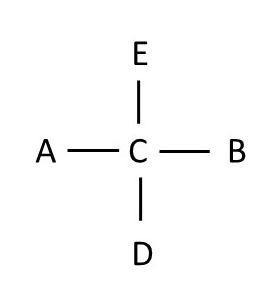
Note that if you rotate a Fischer projection 180 degrees on the plane of the paper, you still have the same molecule, but if you rotate the projection 90 degrees on the plane of the paper, you do NOT get the same molecule. This is because if you simply rotate the molecule 90 degrees on the plane of the paper, the structure no longer follows the rule that groups on the horizontal axis should be in front of the chiral atom, and groups on the vertical axis should lie behind the chiral atom.
Let's explore a molecule with two chiral centers.
Note that when the image above rotates to its final position, chiral center 1 is oriented so that the two groups that are vertical (A and chiral center 2) lie behind the chiral center, and the two groups that are horizontal (B and C) lie in front of the chiral center. Note that although atom 2 has one substituent (C) that lies in front of atom 1, the four atoms that are directly attached to chiral center 1 are in the proper orientation for this Fischer projection. See the simplified Fischer projection below:
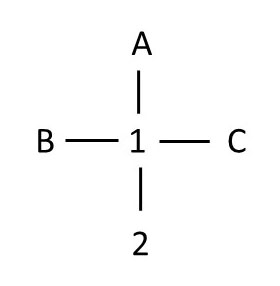 exploring second chiral center
exploring second chiral centerNote that to evaluate the second chiral center, we have to reorient the molecule. Since chiral center 1 lies above chiral center 2 in the Fischer projection, we want to align the molecule with atom 1 above (and behind) atom 2. Here is the partial Fischer projection for chiral center 2:
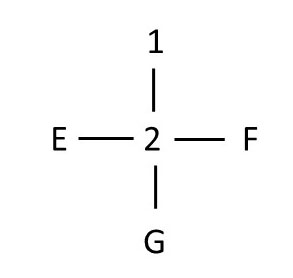
In order to draw the Fischer projection for the ENTIRE molecule with two chiral centers, we have to superimpose the two drawings where adjacent atoms overlap. Here's the Fischer projection of the entire molecule:
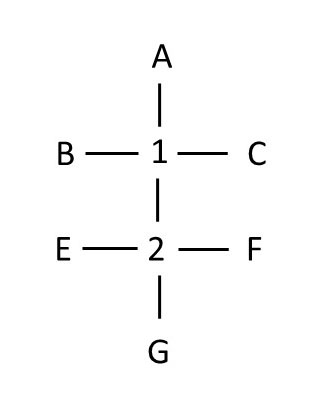
Compare the Fischer projection with the model at right. Note that although chiral center 2 has the 'correct' orientation of the substituents in the Fischer projection, substituents B and C attached to chiral center 1 appear to be backwards. But notice that at chiral center 1, the molecule is oriented to violate Fischer projection rules: atoms lying horizontal to chiral center 1 are BEHIND the chiral center. Thus, to evaluate chiral center 1, we have to rotate the molecule so that B and C are in the front. When we do so, they are in the same location as in the Fischer projection (but now atoms in the second chiral center appear to be 'off'). Thus, each chiral center has to be evaluated individually, then Fischer projections aligned so that two projections overlap on two atoms to draw the whole Fischer projection.
D-glucose has the chemical formula C6H12O6. Click the button below to see the structure of D-glucose. We are using the CPK coloring scheme, in which carbon is gray, oxygen is red and hydrogen is white.
Recall that chiral centers have four different substituents attached to a central carbon. Use your left mouse button to drag and rotate the glucose molecule at the right. How many chiral centers are there in glucose?
2
3
4
5
6
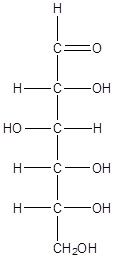
The carbon atoms are numbered sequentially in glucose, beginning with the carbon that has a double-bonded oxygen atom attached. (Carbon atoms are numbered in the Jmol image at right.) Carbon 1 only has three groups attached to it, so, although all three groups are different, because there are not four different constituents, it is not a chiral center. Carbon 6 has four groups attached to it, but because two of the groups are hydrogen, this is also not a chiral center. Carbons 2 and 5 each have four different groups attached to them: H, OH, a one carbon group and a four carbon group. These are chiral centers. Similarly, carbons 3 and 4 have four different groups attached to them: H, OH, a two carbon group and a three carbon group. These are also chiral centers. Therefore there are four chiral centers in D-glucose.
D-glucose orientation 1Note that in orientation one, not all chiral centers are oriented to write Fischer projections. Which chiral centers are in the correct orientation to draw their Fischer projections?
Carbons 2 and 3
Carbons 2 and 4
Carbons 3 and 4
Carbons 3 and 5
Carbons 4 and 5
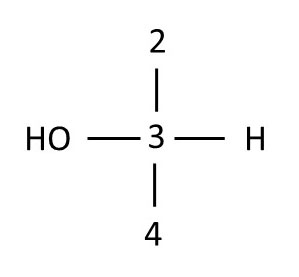
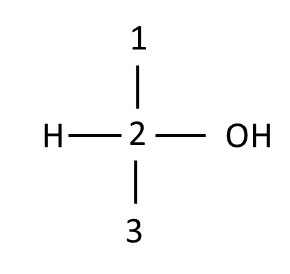
Recall that Fischer projections show groups that are attached to the chrial center on the horizontal axis as protruding toward you and those that are attached to the chiral center on the vertical axis as pointing away from you. Thus, carbons 3 and 5 are in the correct orientation to draw their Fisher projections. Draw the Fischer projections for these two carbons, using numbers to represent carbon atoms and H or OH to represent these groups.
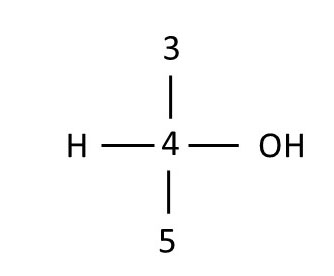
Using D-glucose orientation 2, draw the Fischer projections for chiral centers 2 and 4. Finally, combine the drawings into a Fischer projection for the whole molecule, overlaying common carbon atoms that lie horizontally to form a single carbon chain. Compare your drawings with those below. If your drawings do not match those below, review the images and Fischer projection definitions, then try again.

 Save/Export Your Answers to the Questions in This Jmol Exploration
Save/Export Your Answers to the Questions in This Jmol Exploration