



This Jmol Exploration was created using the Jmol Exploration Webpage Creator from the MSOE Center for BioMolecular Modeling.
Please start by completing the entire Introduction, then the four sections in the CIP4 Case Study in the order indicated by your TA (use the 'Content' bar on the top left of your screen to jump to different sections). After you have completed and answered questions embedded in the entire tutorial, be sure to complete the Final Section at the end.
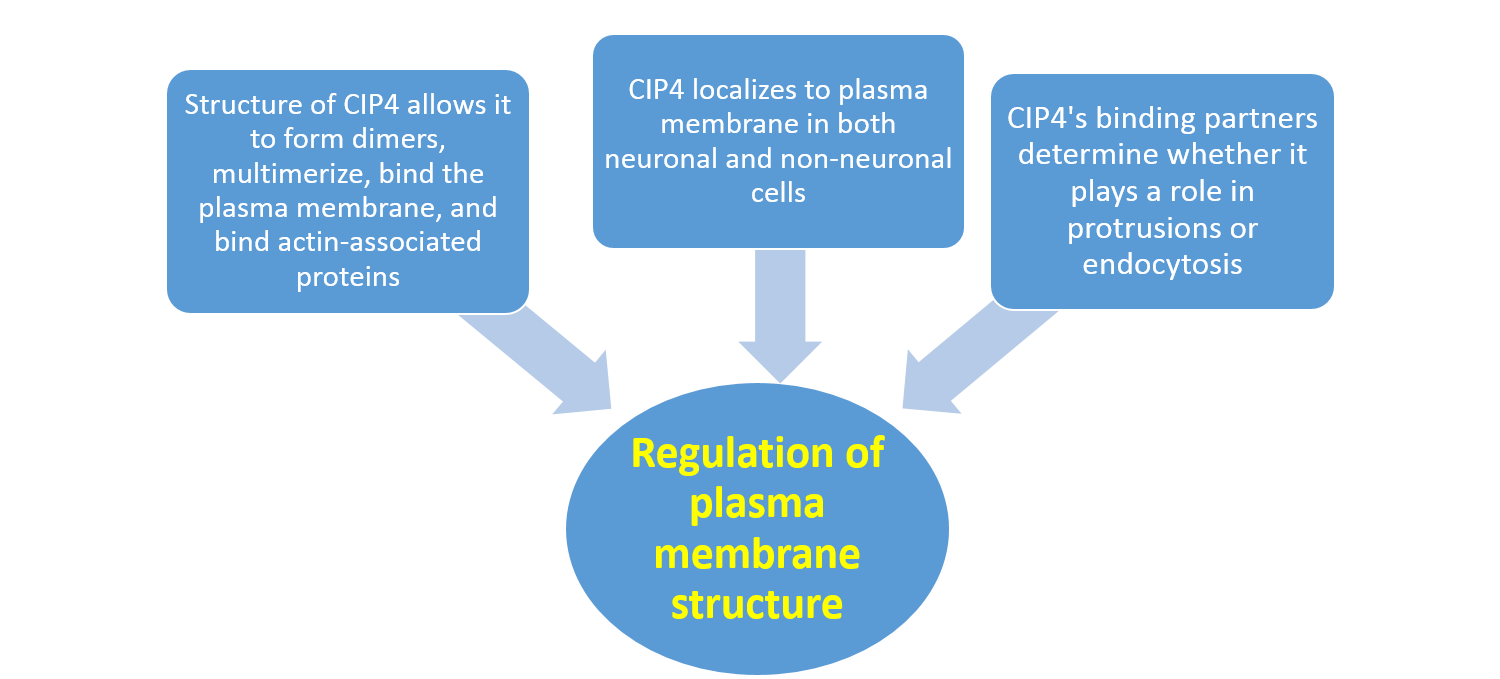
In this tutorial, we will examine the CIP4 protein as an example of how a protein's structure, location, and dynamic interactions with other biomolecules regulate a key cellular function: plasma membrane structure. After completing this tutorial, students will be able to recognize/predict/graph/hypothesize/draw a diagram/design an experiment to demonstrate their understanding of how plasma membrane structure is regulated by CIP4's:
1. Structure, which allows it to: form dimers, multimerize with other CIP4 molecules, bind the plasma membrane, and bind actin-associated proteins
2. Localization to plasma membrane in both neuronal and non-neuronal cells
3. Binding partners, which determine whether it plays a role in protrusions or endocytosis
This section reviews some important terminology. Refer back to this section as needed.
Backbone- this representation shows the peptide chain only, without the side chains (also called residues or R groups). Each kink on a chain represents the central α carbon of one amino acid.
Ball and stick- this representation is used to shows particular amino acid side chains. Each ball and stick color on a sidechain represents a different atom: white for hydrogen, black for carbon, blue for nitrogen, red for oxygen, yellow for sulfur. You do NOT have to memorize these, but note that the specific atoms that compose these side chains determine the properties of the amino acid (polar, nonpolar, negatively charged, etc.) and thus how the protein interacts with other biomolecules!
Remember to interact with the model. Click on the molecule and drag your mouse to spin the molecule. Scroll your mouse to zoom in and out. When you pause your mouse over a kink, a small pop up window will appear with a three-letter identifier for that amino acid (e.g., PRO for proline).
Biomolecular structure, location, and dynamic interactions with other molecules affect cellular functions like the structure of the plasma membrane (see Fig. 1).
This tutorial will summarize the structure and the function of Cdc42 Interacting Protein 4 (CIP4), as a case study (remember you learned a little about this protein in the first Jmol tutorial on protein structure). You should be able to recognize and apply the principles learned from CIP4 to your understanding of other cellular functions.
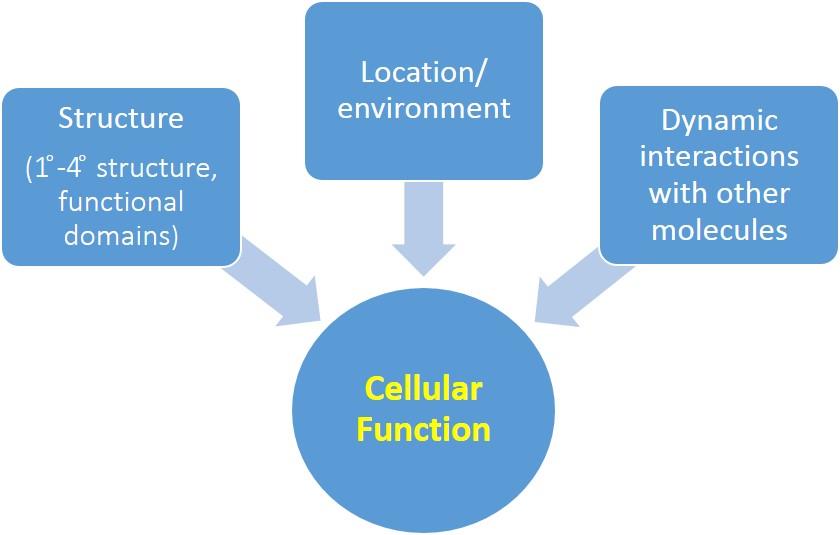
CIP4 is a homodimeric protein, and each of its monomers has three major functional domains: F-BAR, SH3, and HR1 (see Fig. 2). Click the button below to see the homodimer of the F-BAR domain.
In this tutorial, you can view the F-BAR dimer of CIP4 to the right. It is important to remember that you are only viewing the F-BAR domain dimer of CIP4 and not a dimer of the entire protein.
Through multerimerzation (the interaction of multiple CIP4 proteins), CIP4 functions in endocytosis, where it helps to create elongated, tubular vesicles. Recall that CIP4 was one of the molecules in the clickable cellular landscape of endocytosis (Fig. 3).
The primary structure of CIP4 is a linear 545 amino acid sequence of CIP4's three main domains: F-BAR, HR1, and SH3, as well as amino acid sequences between each of these domains.
Based on their primary amino acid sequence, proteins adopt various secondary structures, including α helices and β sheets (β sheets are made of individual β strands). A large amount of CIP4's secondary structure is unknown, because it is unable to be determined with the technology currently available. In Fig. 4, these unknown regions are shown as thin strings connecting the F-BAR, HR1 and SH3 domains. Their length accurately represents the number of amino acids known to span these regions. However, because of the flexible 'arms', in the functioning molecule these regions are probably bent and looped, bringing the HR1 and SH3 domains much closer to the F-BAR domain.
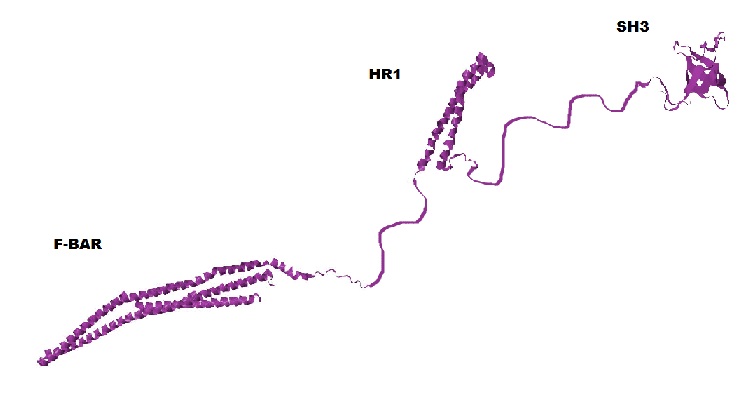
What types of secondary structures do the F-BAR, HR1 and SH3 each adopt?
Interactions between residues (R groups) determined by primary and secondary structure determine tertiary structure (protein folding) and allow for the formation of functional domains.
The F-BAR and HR1 functional domains are α helical bundles that form from the individual α helices, and the SH3 functional domain is a single β barrel that forms from β sheets. The structure of the F-BAR domain was determined through crystallization and X-ray diffraction, while the HR1 and SH3 domains were determined using solution nuclear magnetic resonance (NMR).
Click the button below to see the F-BAR domain.
While this is the structure of a single monomer, it is not a functional unit. To become a fully functional protein, CIP4 first requires quaternary structure. The quaternary structure of CIP4 is a homodimer. Fig. 5a shows one monomer in green and the other monomer in purple and Fig. 5b shows the CIP4 dimer in a rainbow of colors.
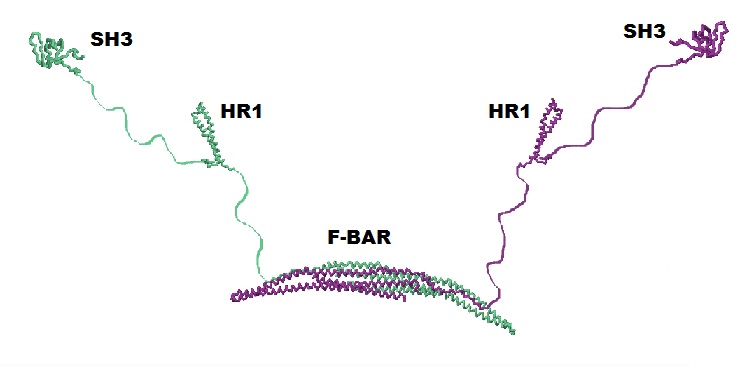
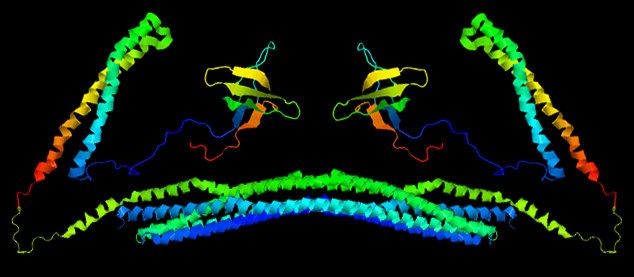
As noted above, the amino acid sequences in between the F-BAR and the HR1 domain and between the HR1 domain and the SH3 domain are flexible. It is hypothesized that these two 'arms' can have different configurations: they may extend out (Fig. 5a and Fig. 2), they may rotate onto the top (convex) surface of the F-BAR dimer (Fig. 5b), or they may rotate onto the bottom (concave) surface of the F-BAR dimer (not shown).
To become a fully functional protein, CIP4 first requires quaternary structure. The quaternary structure of CIP4 is a homodimer.
What does it mean that CIP4 is a homodimer?
Remember that in an aqueous environment, proteins tend to adopt tertiary structure and combine with other proteins (quaternary structure) so that hydrophilic residues tend to be on the _A_ of the protein, where they contact water, while hydrophobic residues tend to be on the _B_ of the protein, where they do not contact water
Click the button below to see the hydrophobic residues within a single F-BAR monomer in spacefill representation, which helps highlight the charge of the amino acid sidechains.
F-BAR monomer: hydrophobic and hydrophilic residues PDB ID: 2efkIn this model, the hydrophobic residues are in yellow and the hydrophilic residues are in blue. Rotate the model to view the locations of the hydrophobic and hydrophobic regions.
Which surface of the F-BAR monomer interacts with another monomer?
Protein quaternary assembly is largely driven by the need to bury hydrophobic regions from the cytoplasm. The surface of the F-BAR domain that forms a dimer contains α helical bundles that are rich in hydrophobic residues (shown in yellow) while the outer side is rich in hydrophilic residues (shown in blue).
To bury the hydrophobic region of the F-BAR domain on each monomer, two monomers come together at these regions to form a dimer. Forming the dimer is favorable because burying these large hydrophobic regions in the dimer contact surface, which becomes the internal part of the dimer, creates a highly stable protein. This causes the F-BAR dimer to adopt a banana-shaped structure.
Click the button below to view a spacefill representation of the F-BAR dimer.
Where are the hydrophobic residues located in this model?
Before CIP4 can function, it not only has to dimerize but also multimerize with other CIP4 proteins. That is, the F-BAR dimers of multiple CIP4 proteins interact with each other at the plasma membrane, for example to form a helical coat that surrounds the invaginating tubular vesicle during endocytosis (Fig. 6).
This multimeric coat is held together by tip-to-tip and lateral interactions between separate F-BAR dimers (see Fig. 7). The lateral interactions are electrostatic bonds between adjacent amino acids that allow the dimers to laterally interact with each other (e.g. lysine 66 (K66) on one dimer with glutamic acid 283/285 (E283/E285) on another dimer, as shown in the center of Fig. 7). Based on these lateral interactions, neighboring dimers should overlap by about half of their length, like partially interlaced fingers. Tip-to-tip interactions are bonds between the amino acids at the tips of each dimer, as shown where K166 is located in Fig. 7.
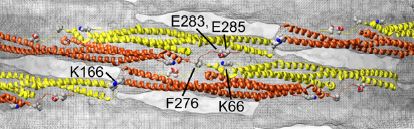
Also important for the lateral interactions between CIP4 dimers are the hydrophobic interactions between the phenylalanine 276 (F276) residues on adjacent F-BAR dimers. When two dimers interact, this allows the hydrophobic F276 residues from adjacent CIP4 dimers to be near one another and be hidden from the surrounding hydrophilic parts of the protein. Mutating this phenylalanine to an aspartate inhibited CIP4 from binding the plasma membrane. Click the button below to view the location of the phenylalaine 276 (F276) on each F-BAR monomer.
F-BAR dimer: phenylalanine (yellow) PDB ID: 2efk.pdb1 (biological assembly)Other lateral interactions include ionic interactions between positively charged residues on one dimer and negatively charged residues on another dimer.
F-BAR dimer: important charged residues PDB ID: 2efk.pdb1 (biological assembly)The positively charged lysines 66 and 273 (K66/K273) are colored red and the negatively charged glutamic acid 285 (E285) and aspartic acid 286 (D286) are colored blue.
What effect would mutating lysine 66 and 273 to glutamatic acid have on F-BAR's interaction with other biomolecules and WHY (hint: what can these lysines no longer do?)
Lysine 166 (K166 in Fig. 7) is the most important amino acid in the tip-to-tip interactions between F-BAR dimers. It forms electrostatic bonds with aspartic acid residues on the tip of an adjacent dimer (not shown in the figure). The mutation of lysine 166 to alanine dramatically compromised CIP4's ability to bind the plama membrane.
F-BAR dimer: lysine 166 (green) PDB ID: 2efk.pdb1 (biological assembly)Based on what you know about the charges of the amino acids, briefly describe why mutating lysine 166 to alanine prevents multimerization.
When thinking about how CIP4 functions at the plasma membrane, recall that membranes are made of phospholipid bilayers. Phospholipids consist of hydrophobic tails and hydrophilic phosphate head groups.
Where are the hydrophobic tails and the hydrophilic heads of the phospholipids located in the bilayer?
Some phospholipid head groups are negatively charged. Two types of head groups that are negatively charged, due to one or two additional phosphate groups, are phosphatidylinositol (4,5)-bisphosphate (PIP2) and phosphatidylinositol (3,4,5)-trisphosphate (PIP3). These phosphoinositides are generally at very low concentrations in the plasma membrane. Phosphatidylinositol (PI) is phosphorylated to produce PIP2 and then phosphorylated again to produce PIP3 only at specific regions of the plasma membrane and at specific times. Thus, in a sense, PIP2 and PIP3 are signaling lipids (i.e. the presence of these negative charges allow signaling cascades to initiate).
What charge do molecules that interact with PIP2 and PIP3 at the surface of the plasma membrane have?
CIP4 has many positively charged amino acid residues on the surface of its F-BAR dimer that allows it to interact electrostatically with PIP2 and PIP3 in the plasma membrane. Click the button below to see the positive residues of the F-BAR dimer.
F-BAR dimer: positive residues PDB ID: 2efk.pdb1 (biological assembly)However, not all of the positive residues located in the F-BAR domain play a role in plasma membrane interactions. Because CIP4 creates tubular vesicles, it is reasonable to predict that the residues important for endocytosis would likely be on the concave surface of F-BAR, allowing it to form the tubular vesicle by constraining the membrane to match the curvature of the dimer.
F-BAR dimer: positive residues important in endocytosis PDB ID: 2efk.pdb1 (biological assembly)Scroll over the F-BAR dimer to identify the charged amino acids. Which amino acids are these?
What would happen if you mutated these positive residues to negatively charged residues?
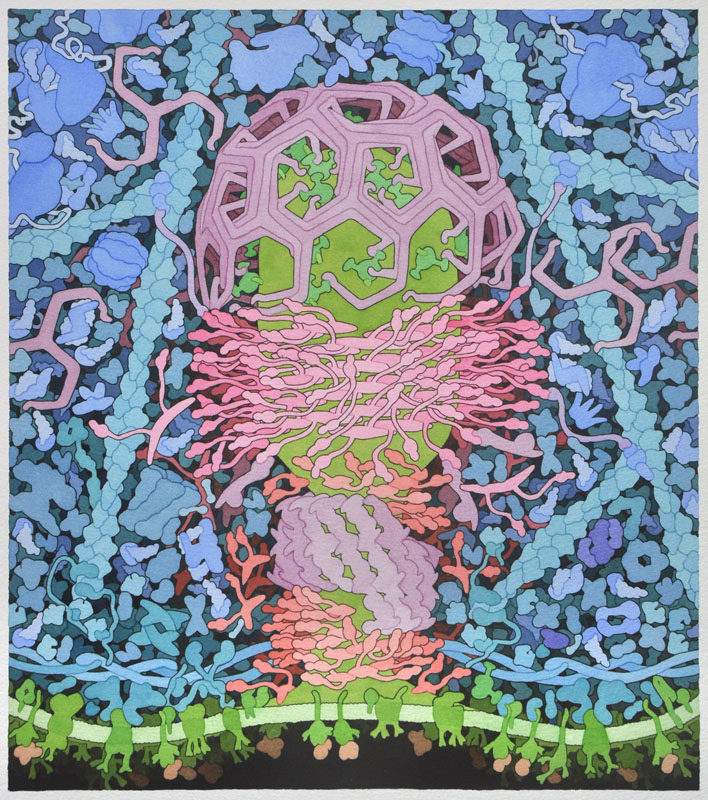
Point mutations of these positive residues were performed to see which ones play a role in endocytosis and which ones do not. Notice that these residues are in four distinct clusters (two clusters on each CIP4 monomer). Upon the mutation of these positive residues, the ability of CIP4 to bind the plasma membrane was compromised. Another study found that these clusters of positively charged amino acids could be accurately superimposed on a 3D reconstruction of a cryo-electron microscopic map of the interaction of CIP4 with curved membrane (Fig. 8), suggesting that these residues do indeed interact with the plasma membrane.
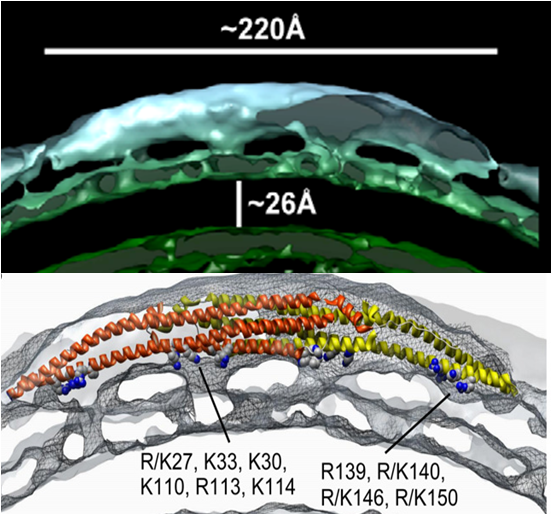
If a cell didn't express any F-BAR proteins what shape of endocytic structures could it form? Assume that F-BAR proteins are the only membrane bending proteins in endocytosis other than clathrin.
As mentioned above the 'arms' of CIP4 (the C-terminal half of the protein composed of the HR1, SH3 and intervening regions) may be floppy and therefore not easily crystalized. How might the floppiness of these arms affect the ability of CIP4 to bind to vesicles, if at all? If you think the arms could affect CIP4 binding can you think of a mechanism (post-translational modification) by which such changes in the arms would be regulated?
As you have seen in this tutorial, in non-neuronal cells CIP4 localizes to the plasma membrane to form long tubular endocytic vesicles. We now know that CIP4 is involved in two different cellular processes at the plasma membrane within different cell types. In early developing neurons, CIP4 localizes to protruding plasma membranes! How CIP4 interacts with the plasma membrane to form protrusions is less well understood. To understand how CIP4 localizes and acts in a context-dependent fashion it will be important to know how the protein functions and how it may be regulated differently in different cell types. In both kinds of cells, CIP4 interacts with polymerizing actin to 'push' the plasma membrane directionally. Recent evidence indicates that protrusions form when CIP4 interacts with a specific set of proteins and lipids while endocytic vesicles are formed when CIP4 interacts with a different set of proteins and lipids (see Fig. 9).
Taking into account CIP4's structure and localization, how might it bind to the plasma membrane during the formation of protrusions?
Putting all of this together, reflect on how CIP4 structure, cellular location, and dynamic interactions with other biomolecules all affect the regulation of plasma membrane structure (Fig. 9).
1. Strausberg,R.L., Feingold,E.A., Grouse,L.H., Derge,J.G., Klausner,R.D., Collins,F.S., Wagner,L., Shenmen,C.M., Schuler,G.D., Altschul,S.F., Zeeberg,B., Buetow,K.H., Schaefer,C.F., Bhat,N.K., Hopkins,R.F., Jordan,H., Moore,T., Max,S.I., Wang,J., Hsieh,F., Diatchenko,L., Marusina,K., Farmer,A.A., Rubin,G.M., Hong,L., Stapleton,M., Soares,M.B., Bonaldo,M.F., Casavant,T.L., Scheetz,T.E., Brownstein,M.J., Usdin,T.B., Toshiyuki,S., Carninci,P., Prange,C., Raha,S.S., Loquellano,N.A., Peters,G.J., Abramson,R.D., Mullahy,S.J., Bosak,S.A., McEwan,P.J., McKernan,K.J., Malek,J.A., Gunaratne,P.H., Richards,S., Worley,K.C., Hale,S., Garcia,A.M., Gay,L.J., Hulyk,S.W., Villalon,D.K., Muzny,D.M., Sodergren,E.J., Lu,X., Gibbs,R.A., Fahey,J., Helton,E., Ketteman,M., Madan,A., Rodrigues,S., Sanchez,A., Whiting,M., Madan,A., Young,A.C., Shevchenko,Y., Bouffard,G.G., Blakesley,R.W., Touchman,J.W., Green,E.D., Dickson,M.C., Rodriguez,A.C., Grimwood,J., Schmutz,J., Myers,R.M., Butterfield,Y.S., Krzywinski,M.I., Skalska,U., Smailus,D.E., Schnerch,A., Schein,J.E., Jones,S.J. and Marra,M.A. 2002. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proceedings of the National Academy of Sciences 99(26): 16899-16903
2. Shimada A, Niwa H, Tsujita K, Suetsugu S, Nitta K, Hanawa-Suetsugu K, Akasaka R, Nishino Y, Toyama M, Chen L, Liu ZJ, Wang BC, Yamamoto M, Terada T, Miyazawa A, Tanaka A, Sugano S, Shirouzu M, Nagayama K, Takenawa T, Yokoyama S. 2007. Curved EFC/F-BAR-domain dimers are joined end to end into a filament for membrane invagination in endocytosis. Cell 129: 761-772
3. Miyamoto, K., Tomizawa, T., Koshiba, S., Inoue, M., Kigawa, T., Yokoyama, S. To be Published. Solution structure of the SH3 domain of the Cdc42-interacting protein 4.
4. Kobashigawa, Y., Kumeta, H., Kanoh, D., Inagaki, F. 2009. The NMR structure of the TC10- and Cdc42-interacting domain of CIP4. Journal of Biomolecular NMR 44: 113-118
5. Masuda, M. and Mochizuki, N. 2010. Structural characteristics of BAR domain superfamily to sculpt the membrane. Seminars in Cell and Developmental Biology 21: 391-398
6. Frost A, Perera R, Roux A, Spasov K, Destaing O, Egelman EH, De Camilli P, Unger VM. 2008. Structural basis of membrane invagination by F-BAR domains. Cell 132: 807-817
Save/Export Your Answers to the Questions in This Jmol Exploration