

This Jmol Exploration was created using the Jmol Exploration Webpage Creator from the MSOE Center for BioMolecular Modeling.
The total subunits that Fumarase holds is four. The subunits are crystallized individually. What is shown in the picture below is the four subunit enzyme known as Fumarase. The diagram to the right represents the subunits that have been crystallized from E.coli.
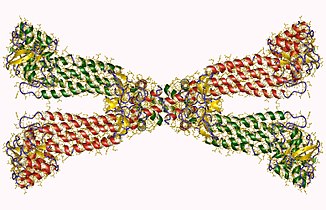
In this diagram, two different types of secondary structures are being shown. β-sheets and α-helices are both present within this representation. β-sheets are represented by the color green. α-helices are represented by the color red. β-sheets and α-helices both represent hydrogen bonding forces within the secondary structure. These forces show the difference between intermolecular forces between the backbone structure which allow protein folding to occur. The protein folding is represented in the following diagram.
secondary structureFor each of the four subunits for Fumarase, the primary structure consists of 456 amino acid sequences.
The active site is represented above in a diagram with the colors blue and red. The red represents the Histidine 188 active site in an individual unit. The color blue represents Lysine 324 in an individual unit. Due the the presences of two subunits, there are four active sites present.
.png)