
Let's talk about blood clotting.
Oxygen and other essential nutrients move throughout our bodies in the circulatory system. The blood. It is a fast and simple way for our cells and organs to get the things they need to survive.
...but there are challenges! While the circulatory system can transport nutrients, it can also carry infections to all areas of the body. This is why it is so important for the antibodies of our immune system to be able to fight this potential danger.
Another challenge that our circulatory system may face is that which is related to the blood. As we know, we must maintain our blood volume in order to live. If we have any leakage of blood out of the circulatory system, we are at risk for losing that blood!
Good thing our bodies have an emergency repair system for such challenges. THE CLOTTING CASCADE."
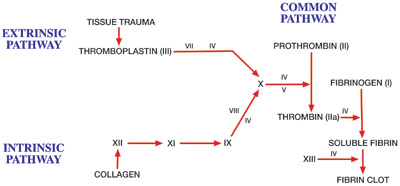
Notice in this image: a bunch of coagulation factors (labeled in Roman Numerals) are responsible for activating the Thrombin precursor, Prothrombin. Thrombin is now activated and functional.
"
The backbone of Thrombin, the green portion, seems chaotic. But it is not!
The amino acids of the protein are in a specific order. This gives the PRIMARY STRUCTURE of Thrombin.
"
Let's look at SECONDARY STRUCTURE now.
Secondary structure involves the backbone of the protein. Hydrogen-Bonding interactions allow the protein backbone to curl up and form alpha helices. Additionally, the backbone can fold to form 'rows' called beta sheets. This promotes the formation of the unique structure we see in the molecule to the right.
Click the button to highlight the differences. The alpha helices are shown in purple, and the beta sheets are shown in orange. "
The next level of protein structure is tertiary structure.
Notice how (in the previous images) that the structure of this protein has a globular shape. This is a compact shape that differs from proteins with a fibrous shape. See the image below. The general structure of fibrous proteins is on the left. The general structure of globular proteins (Thrombin) is on the right."

Unlike with the secondary structure that involved the backbone of the protein, this level of structure has to do with the side chains of that backbone. The non-polar side chains of its amino acid backbone face inwards/inside the globular protein to avoid the aqueous environment. Keep in mind that, for Thrombin, the aqueous environment is the water and blood that runs through our bodies.
The polar side chains of the amino acid backbone face outwards/outside of the globular protein so that it can interact with our blood and circulatory system."

This image shows the nature of tertiary structure. That is, it shows how the side chains of the amino acid backbone interact with each other causing the protein to fold in a specific and unique manner. The protein backbone is labeled. All of the other labeled interactions promote the formation of tertiary structure.
There are several ways in which these side chains can interact with one another. This includes: hydrophobic interactions, salt bridges, hydrogen-bonding, and disulfide bonds.
Click the button below to see the tertiary structure of Thrombin!"
In yellow, we see the hydrogen-bonding interactions that occur between the side groups. The navy blue shows the disulfide bonds that occur between the amino acid, cysteine.
"

This image depicts the formation of a disulfide bond. Two Cysteine amino acids come bond together at their Sulfur atoms."
One last thing about structure!
Specific to Thrombin, it has two different chains of amino acids. It has two α (alpha) chain subunits. This means that it has one heavy alpha chain and one light alpha chain in its structure.
This is a very unique feature about Thrombin! Not all proteins have more than one chain! This is called QUARTERNARY STRUCTURE. Click the button to see the differences. The heavy chain is red. The light chain is aqua blue.
"
This video reiterates what was discussed in the beginning of this tutorial. Coagulation factors help activate Thrombin so it can assist our body's platelets in forming a clot.
How does it do this? Well, Thrombin is a protein that functions as an enzyme. So, once Thrombin is activated by coagulation factors, it binds fibrinogen to its active site.
When this happens, fibrin is created, and fibrin will form strands that will attach to platelets to strengthen and stabilize the blood clot. This is emphasized and shown in the end of the video.
"
The colored portion of this molecule depicts the active site of Thrombin. The three amino acids that are highlighted will interact with fibrinogen to make fibrin. This results in clot formation.
"
What is Thrombin Time (TT)?
As we just saw, Thrombin converts fibrinogen to fibrin. Therefore, the TT reflects this conversion. Interestingly, the TT is also sensitive to inhibitors of this conversion, and these inhibitors may be medications like Heparin & Warfarin.
Human thrombin is taken from the individual and added to plasma. The time taken for the formation of a fibrin clot represents the TT. "
What is Prothrombin Time (PT)?
It is a blood test that measures how long it takes blood to clot. This is very similar to TT, but this test depends on prothrombin. It can help to test for bleeding issues or medication effectiveness.
Prothrombin is the precursor to Thrombin, and Vitamin K is needed to synthesize prothrombin. The PT test determines the presence of prothrombin. In addition, it looks for the presence of other clotting factors.
"
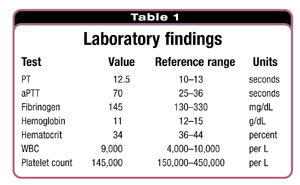
If the times are elongated, this signifies an increase in bleeding.
Several things can lengthen PT:
1. deficiency of clotting factors
2. lack of Vitamin K
3. liver disease
4. disseminated intravascular coagulation (DIC)
5. medications (warfarin, heparin, aspirin)
"

Remember: if we bleed, our bodies are smart and can stop it! However, certain diseases/conditions/wounds impair our ability to clot. This requires further intervention.
For the majority of cases, Thrombin and the Clotting Cascade can help us maintain our blood volume. "