



This Jmol Exploration was created using the Jmol Exploration Webpage Creator from the MSOE Center for BioMolecular Modeling.
Fibronectin is a glycoprotein of the extracellular matrix that binds to membrane-spanning receptor proteins. It has a high molecular weight of about 440 kDa. Fibronectin binds the cell to the extracellular matrix and organizes it. It can bind collagen, fibrin, and proteoglycans. This protein is also involved in cell adhesion, growth, migration, differentiation, and wound healing (Pankov & Yamada, 2002). There are two different types of fibronectin present in vertebrates which include soluble plasma fibronectin and insoluble cellular fibronectin (Pankov & Yamada, 2002).
Soluble plasma fibronectin is produced by hepatocytes in the liver and is a major component of blood plasma. Insoluble cellular fibronectin is secreted by many cells in the body, mainly fibroblasts, and is a major part of the extracellular matrix (Pankov & Yamada, 2002). This tutorial shows a fragment of the fibronectin protein.
Color Scheme:
Carbon- White
Nitrogen- Purple
Oxygen- Light Blue
Sulfur- Pink
Zinc- Green
Domains
There are three domains in the entire protein of fibronectin. Since this is only a fragment of the protein, we can only see two domains in this image. We can identify these two domains as type 1 and type 2 because they are stabilized by disulfide bonds, which we will see later in the tutorial.
Fibronectin exists as a protein dimer, consisting of two nearly identical polypeptide chains, or monomers. These chains are linked by a pair of C-terminal disulfide bonds (Mao & Schwarzbauer, 2005). Each subunit of the protein contains three modules: type 1, type 2, and type 3 which are all composed of two anti-parallel β- sheets (Mao & Schwarzbauer, 2005). Types 1 and 3 contain disulfide bonds, however, type 2 does not contain these bonds which allows it to unfold partially from applied forces.
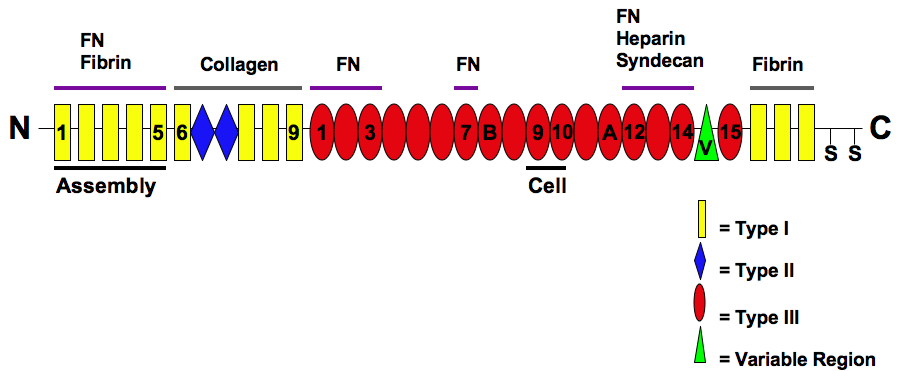
The three types of modules are arranged into different protein-binding domains along the fibronectin monomer (Mao & Schwarzbauer, 2005). There are four different fibronectin-binding domains, which allow fibronectin to interact with other fibronectin molecules. The first domain is called the assembly domain and it is required for the initiation of fibronectin matrix assembly (Mao & Schwarzbauer, 2005). The protein also contains domains for fibrin-binding, collagen-binding, fibulin-binding, heparin-binding, and syndecan-binding (Mao & Schwarzbauer, 2005).
The primary structure, or the main sequence, is the order in which the amino acids are connected to one another (McKee & McKee, 2014). Fibronectin is 308 residues long.
Color Scheme: CPK
Secondary structure is a combination of α-helices and β- sheets. The formation of α-helices and β-sheets occurs due to the pattern of hydrogen bonding forces between the backbone atoms of proteins. In this structure the proteins will fold themselves to form these various structures due to interaction between the atoms of the backbone and amino acid residues (McKee & McKee, 2014).
Helical: 2 α-helices, 8 residues
In an α-helix, the backbone of the protein forms intramolecular hydrogen-bonding forces that cause the protein to fold in a helical shape. These interactions occur between the Nitrogen (N-H) and the Oxygen (C=O). These interactions cause a right handed coil or spiral known as a helix (McKee & McKee, 2014).
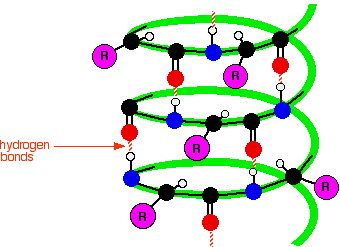
Color Scheme:
α-helix- red
45% Beta Sheets: 38 strands, 140 residues
In a β-pleated sheet, a row of amino acids residues can form hydrogen bonding interactions with a second row of amino acids. Typically β-sheets are 3-10 amino acids long with the backbone in extended conformation.(McKee & McKee, 2014).
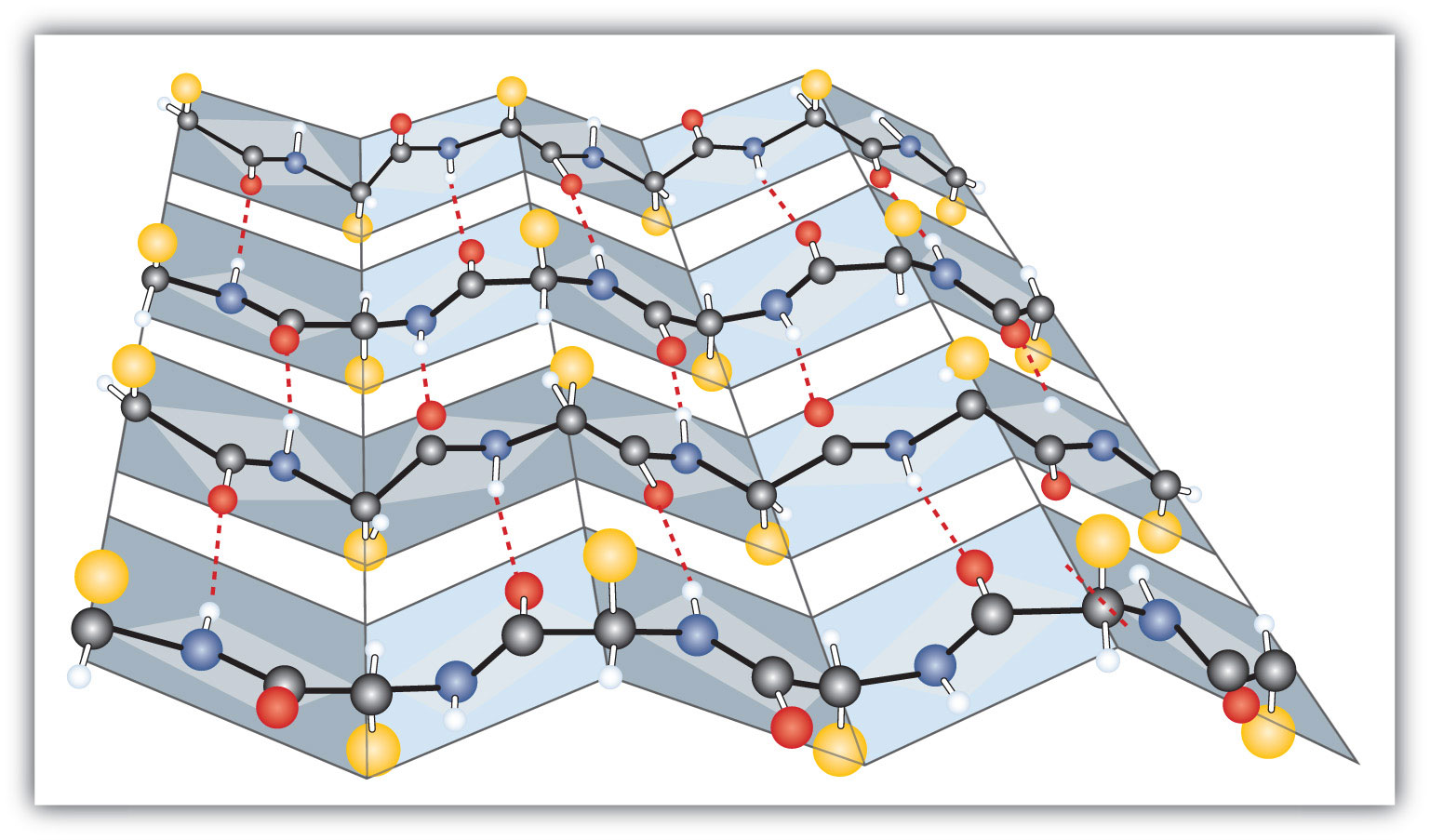
Color Scheme:
β-sheets- Purple
Fibrous
Fibronectin is a fibrous or scleroprotein. Fibrous proteins have a three-dimensional shape of a rod or wire. This shape occurs because of the folding of the protein due to interactions between the amino acid residues and the backbone. These interactions typically contain disulfide bonds. Fibrous proteins are generally insoluble in water and denature less easily that globular proteins (McKee & McKee, 2014).

Blue areas indicate areas of the protein that are more stable while red areas indicate more 'wiggly' areas that are more unstable.
Zinc
In the extracellular matrix, zinc is required for the activity of matrix protease enzymes, which help to remove and remodel glycoproteins that are required in development and wound healing (Askari et al., 2012).
Disulfide bonds
Single covalent bonds between sulfur atoms. This linkage is also called an SS-bond or a disulfide bridge. Within fibronectin the disulfide bond occurs between two amino acids called cysteine that contain the thiol functional groups. Disulfide bonds are important in protein folding and creates stability. These interactions typically take place in proteins that are located outside the cell as the cytosol contains reducing elements (Sevier & Kaiser, 2002).

Hydrogen Bonds
Hyrodgen bonding occurs between polar groups containing Oxygen, Nitrogen, or Fluorine bonded to Hydrogen due to electrostatic charges. These interactions gives fibronectin its three-dimensional shape. The hydrogen bonding between the backbone atoms creates secondary structure of proteins. The hydrogen bonding interactions that occur between the amino acid residues creates the tertiary shape of fibronectin which is fibrous (McKee & McKee, 2014).
Fibronectin has many roles that help cells to function normally. Insoluble cellular fibronectin separates and supports the organs and tissues of an organism (Pankov & Yamada, 2002). The protein is involved in cell adhesion, growth, migration, differentiation, and even wound healing. Soluble plasma fibronectin plays a crucial role in wound healing by depositing itself at the site of injury and forming a blood clot to stop bleeding (Grinnell, 1984). Fibroblasts and macrophages remodel the injured area and degrade the proteins that that form the blood clot and replace them with a matrix that very closely resembles the normal tissue of that area. Fibroblasts secrete proteases that will digest plasma fibronectin into fragments and promote wound contraction. This fragmentation of fibronectin exposes its variable region (or V-region) which contains the integrin-binding site. Exposing this region allows for the binding of integrin-expressing cells, which allows fibronectin to adhere to the matrix. Fibroblasts will also then secrete cellular fibronectin and assemble it into an insoluble matrix (Grinnell, 1984).

Decreases in fibronectin expression and increases in fibronectin degradation have been linked to morphological changes in tumor cell lines (Haynes, 1990). Lung carcinoma development has been shown to involve increases in fibronectin expression. Fibronectin can stimulate the vertebrate androgen receptors which increases cyclin D responsible for gene regulation in the cell cycle. Due to the increase in cyclin D expression fibronectin can promote tumor growth (Hans, et al., 2006).

Fibronectin is important in wound healing. Fibronectin is typically located in the extracellular matrix of adult tissues. During the formation of a blood clot Factor XIII is responsible for the covalent cross-linking between fibrin and fibronectin (Mosher, 1975). Decreases in fibronectin levels in plasma have been observed in acute inflammation cases or surgical trauma patients. This reduction of fibronectin levels lead to disseminated intravascular coagulation (Bruhn & Heimburger, 1976). Fibronectin also stimulates cytokines to signal phagocytosis by fibroblasts and macrophages in the damaged tissue. New collagen deposits are also stimulated by fibronectin. This collagen works in conjunction with the fibronectin in order to close wounds (Engvall, et. al., 1978).
Askari, J., Thornton, D., Humphries, J., Buckley, P., & Humphries, M. (2012). THE ALTERNATIVELY SPLICED TYPE III CONNECTING SEGMENT OF FIBRONECTIN IS A ZINC-BINDING MODULE. Matrix Biology. Volume 26(6), 485-493. Retrieved from doi: 10.1016/j.matbio.2007.04.001
Bruhn, H., Heimburger, N., (1976). 'Facotr-VIII-related antigen and cold-insoluble globulin i leukemias and carcinomas'. Haemostasis 5 (3): 189-192
Engvall, E., Ruoslahti, E., Miller, E., (1978). 'Affinity of fibronectin to collagens of different genetic types and to fibrinogen' The Journal of Experimental Medicine 147 (6): 1584-1595
Grinnell, F. (1984). Fibronectin and wound healing. Journal of Cellular Biochemistry. Volume 26(2), 107-116. Retrieved from DOI: 10.1002/jcb.240260206
Han, S., Khuri, F., Roman, J., (2006). Fibronectin stimulates non-small cell lung carcinoma cell growth through activation of Akt/mammalian target of rapamycin/S6 kinase and inactivation of LKB1/AMP-activated protein kinase signal pathways. Cancer Research 66 (1): 315-23. doi: 10.1158/0008-5472.CAN-05-2367
Hynes, R., (1990). Fibronectins. Berlin: Springer-Verlag. ISBM 0-387-97050-9.
Mao, Y. & Schwarzbauer, J. (2005). Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biology. Volume 24(6), 389-399. Retrieved from doi:10.1016/j.matbio.2005.06.008
McKee, J. & McKee, T. (2014). Biochemistry; The Molecular Basis of Life. Oxford University Press, Inc.
Mosher, D., (1975). 'Cross-linking of cold-insoluble globulin by fibrin-stabilizing factor'. The Journal of Biological Chemistry 250 (16): 6614-6621
Sevier, C., Kaiser, C., (2002). 'Formation and transfer of disolphide bonds in living cells.' Nature Revies Molecular Cell Biology. 3 (11): 836-847
Pankov, R. & Yamada, K. (2002). Fibronectin at a glance. Journal of Cell Science. Volume 115, 3861-3863. Retrieved from doi: 10.1242/jcs.00059
Figure 1. https://upload.wikimedia.org/wikipedia/commons/6/66/The_Modular_Structure_of_Fibronectin_and_its_Binding_Domains.png
Figure 2. http://www.rcsb.org/pdb/explore/remediatedChain.do?structureId=3M7P ¶ms.annotationsStr=Site%20Record,DSSP&chainId=A
Figure 3. https://www.chemguide.co.uk/organicprops/aminoacids/ahelix.gif
Figure 4. https://2012books.lardbucket.org/books/introduction-to-chemistry-general-organic-and-biological/section_21/51229446d0888134f4a9856b8213c864.jpg
Figure 5. https://amit1b.files.wordpress.com/2008/03/collagen.png
Figure 6. https://upload.wikimedia.org/wikipedia/commons/2/22/Cystine-skeletal.png
Figure 7. https://ankiweb.net/shared/mpreview/3282835125/0.png
Figure 8. https://www.rarecoagulationdisorders.org/wp-content/uploads/2015/08/figure1.png
Figure 9. https://www.nature.com/nrurol/journal/v11/n3/images/nrurol.2014.15-f1.jpg
Figure 10. https://www.youtube.com/watch?v=f9kQ4MQNMy0